
STEM Scientific and Technical Writing is taught by Dr. Crowthers. In STEM we learn how to properly research, read, and write technical, scientific documents. From August-February we brainstorm, research, and work on our own independent research projects, and present them at February fair in the WPI Odeum. This year's February fair is on Thursday, Feb 16th. Scroll down for more information on my independent research project.
The world has a plastics recycling problem. Over 450 million tons of plastic waste are produced every year, and 76% of this waste ends up in landfills. Only 40.5 tons of plastic are mechanically recycled, a process that is labor-intensive and environmentally harmful in itself. Hydrothermal liquefaction is a newly developed method of plastics recycling that converts waste plastics into energy-dense oils with consumer-use applications. However, hydrothermal liquefaction (HTL) is not viable at a mass scale due to its high energy input and time consumption. The use of catalysts in HTL has been researched as a way of reducing the time and energy it takes to convert plastics to oils. Hydrogen peroxide has seen great success with lowering the reaction time and temperature of polystyrene, but it is currently unknown why hydrogen peroxide works so effectively. In this project, I used a monomer of polystyrene- styrene as a model compound to examine the effects of H2O2 on a smaller scale. This project will bring scientists steps closer to understanding how hydrogen peroxide works and how it can be applied to other plastic wastes.
Recently, scientists have turned to hydrothermal liquefaction as an effective method of plastics recycling with a high amount of energy recovery in the form of oil. Tweaking the HTL process using chemical catalysts has become crucial in bringing HTL to a mass-scale, however it is unknown how some catalysts are able to be so effective.
The goal of this project is to analyze the different reactions that occur when using styrene monomers and hydrogen peroxide in order to determine where oxidation occurs and if hydrogen peroxide is capable of inhibiting the polymerization of plastics.

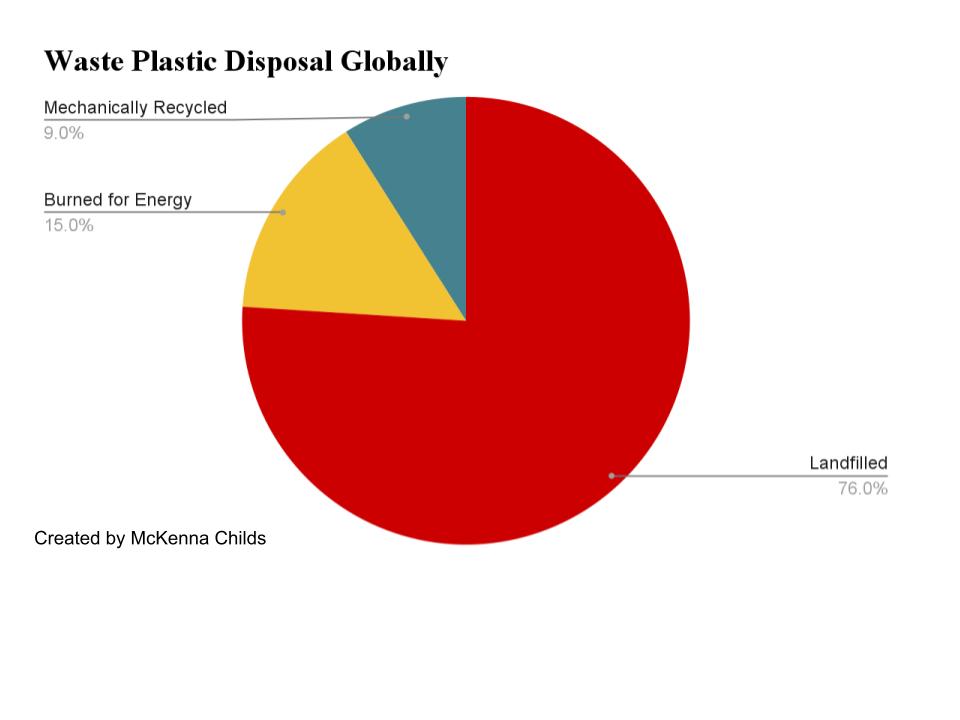
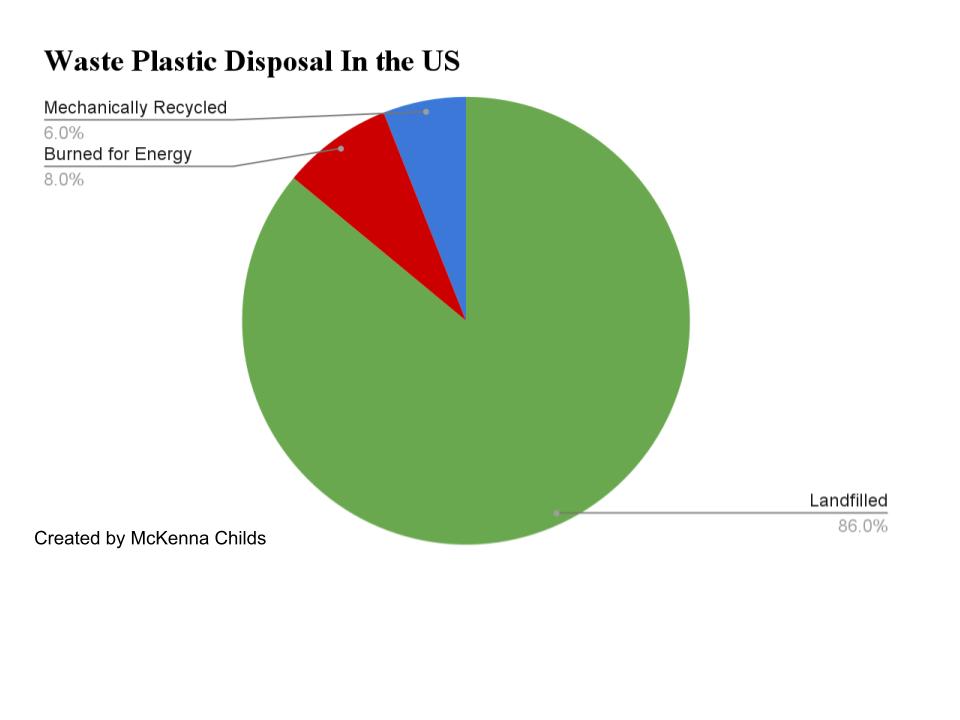
Mechanical recycling is the most common and widely used method of plastics recycling in the world. Plastics are collected from bins and recycling centers and shipped overseas, where they are separated, machine-ground, washed, and compounded. This process is not only incredibly time consuming, but also environmentally risky and overall ineffective. Separation and machine operation requires intense labor. Shipment overseas on boats is expensive and risks emissions as boat fuels burn and contribute to plastic waste if waste falls off or is picked at by seabirds (Andres et al., 2012). Most waste plastics are landfilled, with just 9 wt% being mechanically recycled and 15 wt% being burned for energy recovery (Anshassi et al., 2019). Over 76% of our waste plastics are being landfilled, which makes all of the time and effort spent attempting to recycle futile.
There have been many proposed ways to deal with waste plastics- one of which is hydrothermal liquefaction (HTL). Hydrothermal liquefaction is a chemical process where wet organic matter (food wastes, sewage sludge, agricultural waste, plastics, and more) are placed in a high heat and high pressure environment. During this process, matter breaks down into its building blocks and creates biocrude- or a biologically derived oil. These oils are energy dense and have a wide span of applications- from paint stripper to jet fuel. This has made hydrothermal liquefaction an incredibly promising field for plastics recycling as it is able to harvest energy from typically discarded products.

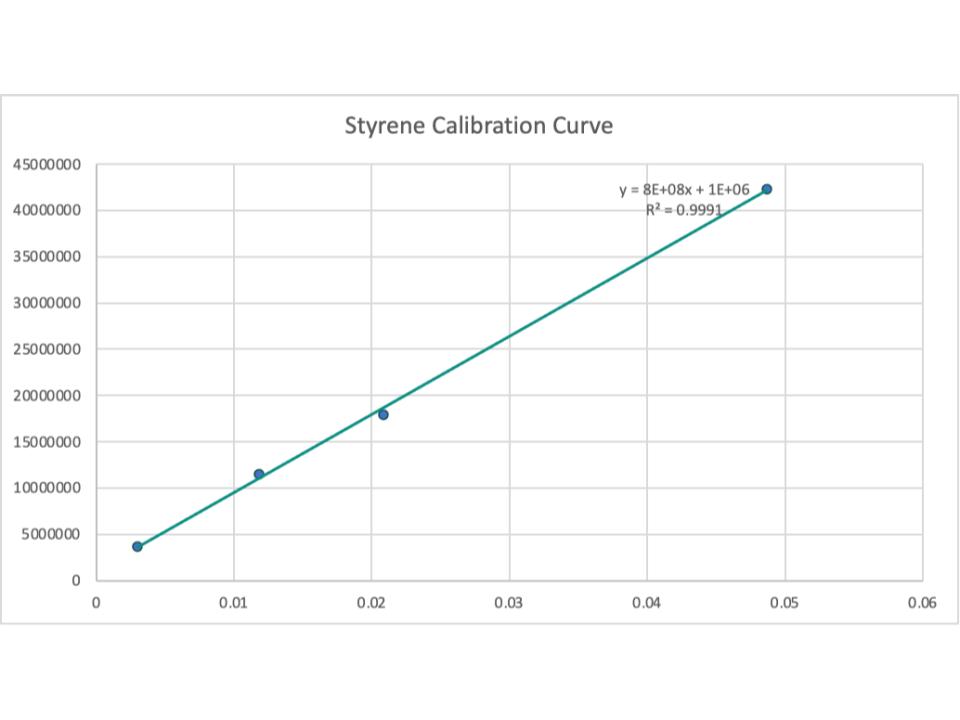
Samples were measured and placed into the reactor in order of least-most volatile in order to prevent any loss in the form of gasses. The reactor was sealed, set up, and placed at 350ºC. The time it took for the reactor to reach its ideal temperature varies depending on the addition of hydrogen peroxide. Reactions ran for 20 minutes each, and once finished were quenched with a mixture of ice and tap water until the reactor was cool enough to move. Liquid samples were poured out into glassware and placed in a plastic centrifuge tube and centrifuged for 5 minutes at 3500 rpm until oil phase was fully separated from aqueous phase. The aqueous solution was then extracted from the centrifuge tube by hand using a glass pipette. Samples were placed in an alternate glass container, labeled, and stored. Oil samples were measured and placed in a smaller glass container for further gas chromatography analysis. Chemicals were collected and analyzed using gas chromatography - mass spectrometry. Data from each sample was imported into a computer program and plotted. From the height and area of peaks as well as the recommendations given by the gas chromatography system, chemicals were identified and plotted on the graph. Components of each chemical were compared to each other by looking at the graph data.
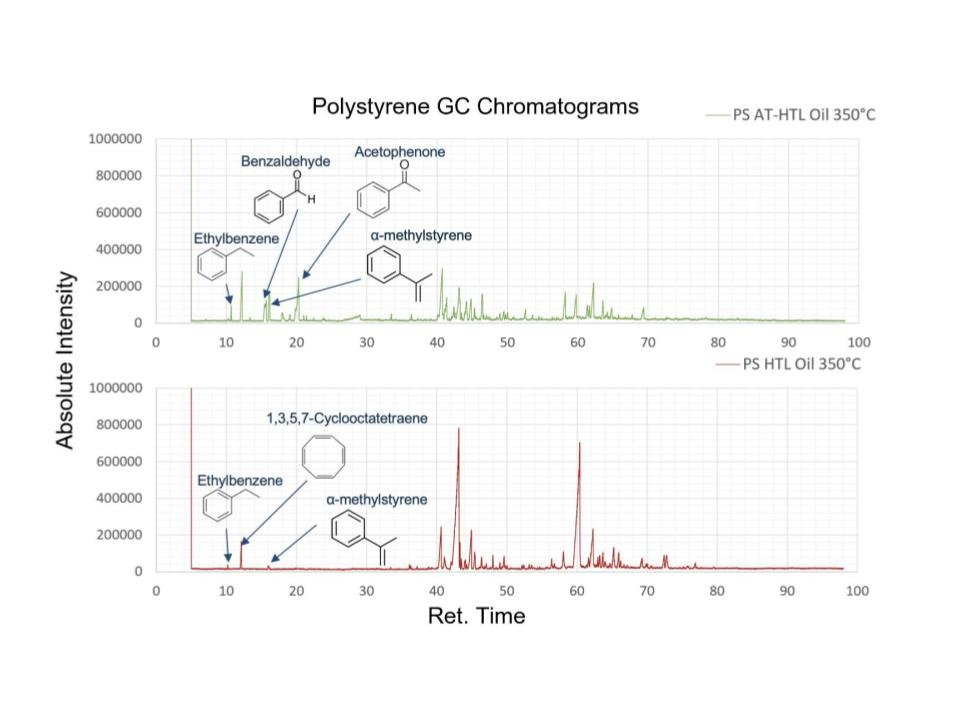


When styrene undergoes HTL without H₂O₂ it polymerizes and produces a significant amount of hard solid, likely polystyrene. It also produces a deep-yellow oil with moderate viscosity. The composition of the oil is primarily styrene, as well as 1-Propene,3-(2-cyclopentenyl)-2-methyl-1, 1-diphenyl-1 2-Diphenyl-1-isocyano ethane, and benzonitrile,m-phenethyl-. The compounds are primarily subunits of polystyrene, and none are oxidized.
When HTL of styrene occurs with H₂O₂ it produces a small amount of soft, waxy, deep brown solid and a viscous dark oil similar to balsamic vinegar. The oil is primarily composed of styrene, Benzaldehyde, and alpha-Methylstyrene, some of which are oxidized compounds.
Based on the chemical composition of the oil created from HTL with hydrogen peroxide it is reasonable to conclude that hydrogen peroxide is capable of reacting with monomers oxidizing monomers and subunits. The amount of oil retrieved as well as the consistency and amount of solid suggests that hydrogen peroxide either partially or completely inhibits the re-polymerization of styrene into polystyrene.
The applications and further experiments created by the results of this experiment are endless. Now that it is known that hydrogen peroxide has a capability of reacting with styrene, further breaking it down and oxidizing its compounds, preventing re-polymerization, it may be worthwhile to consider hot-injection of hydrogen peroxide as a new and effective method of increasing polystyrene oil production.
Additionally, HTL is a time consuming and energy-inefficient process. Hydrogen peroxide has the capability to reduce reactor startup time and overall run time, all while increasing the total oil yield. This brings HTL steps closer to becoming a viable alternative to current methods of recycling, as lowered amounts of energy input and time are necessary.