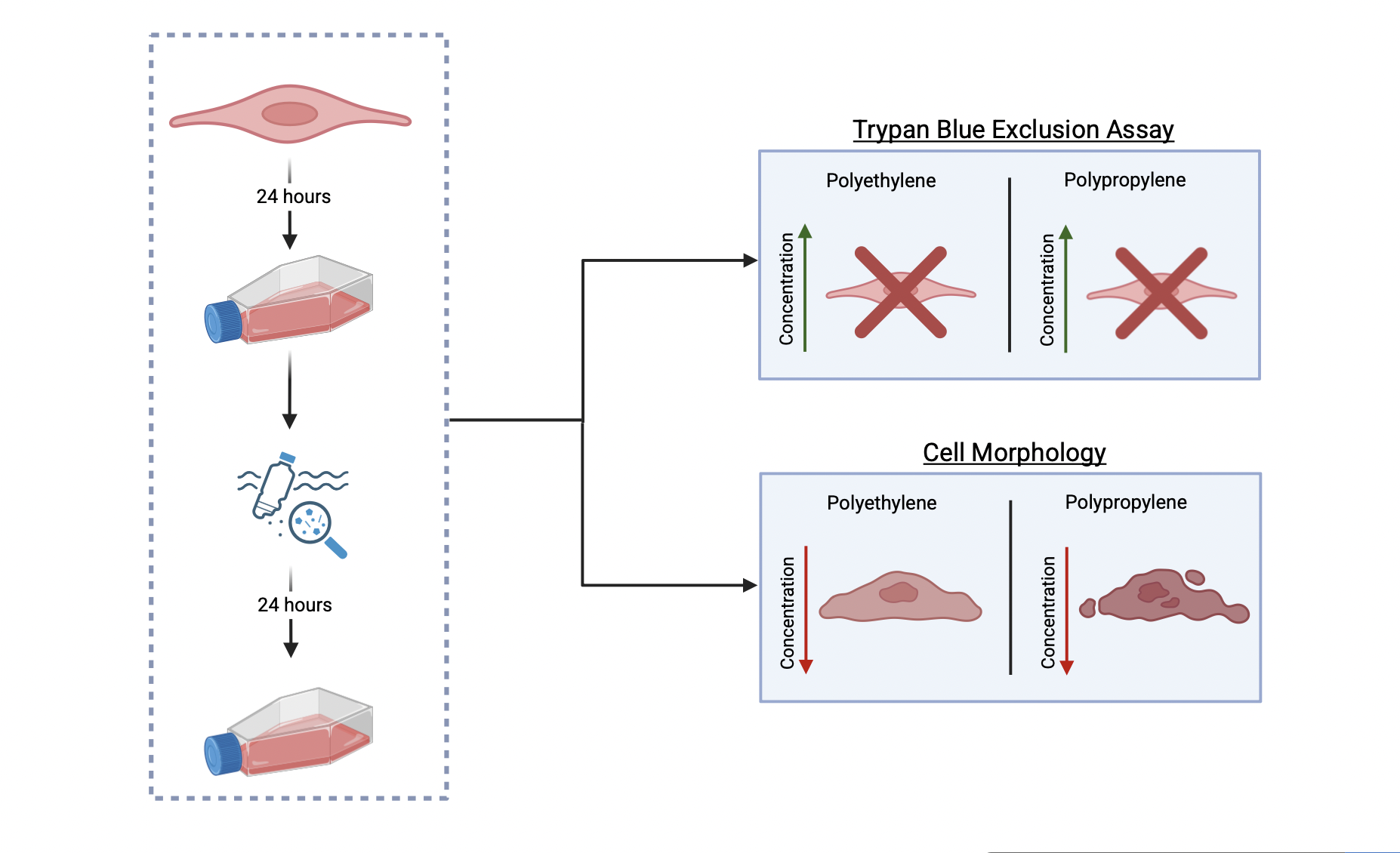
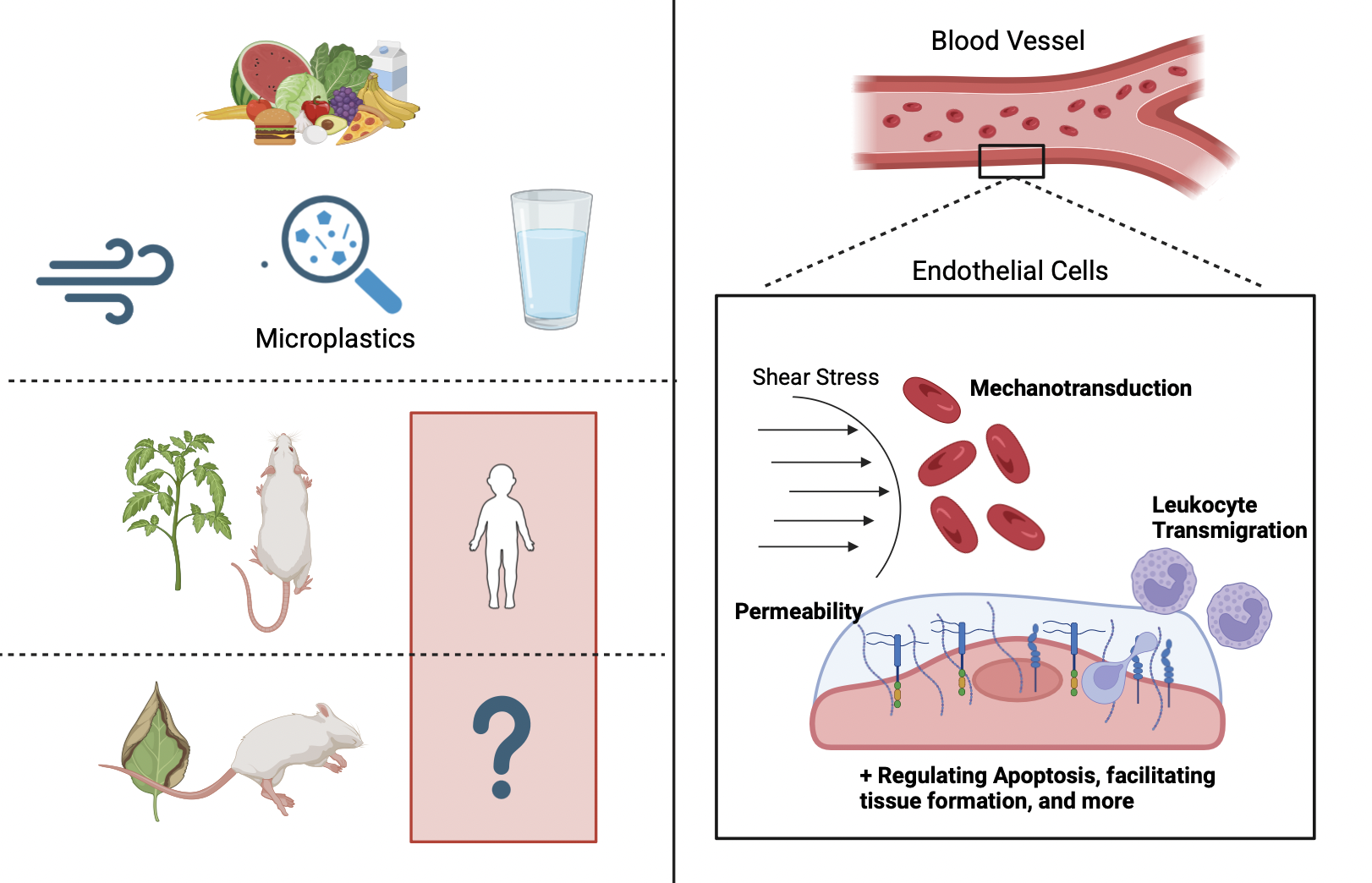
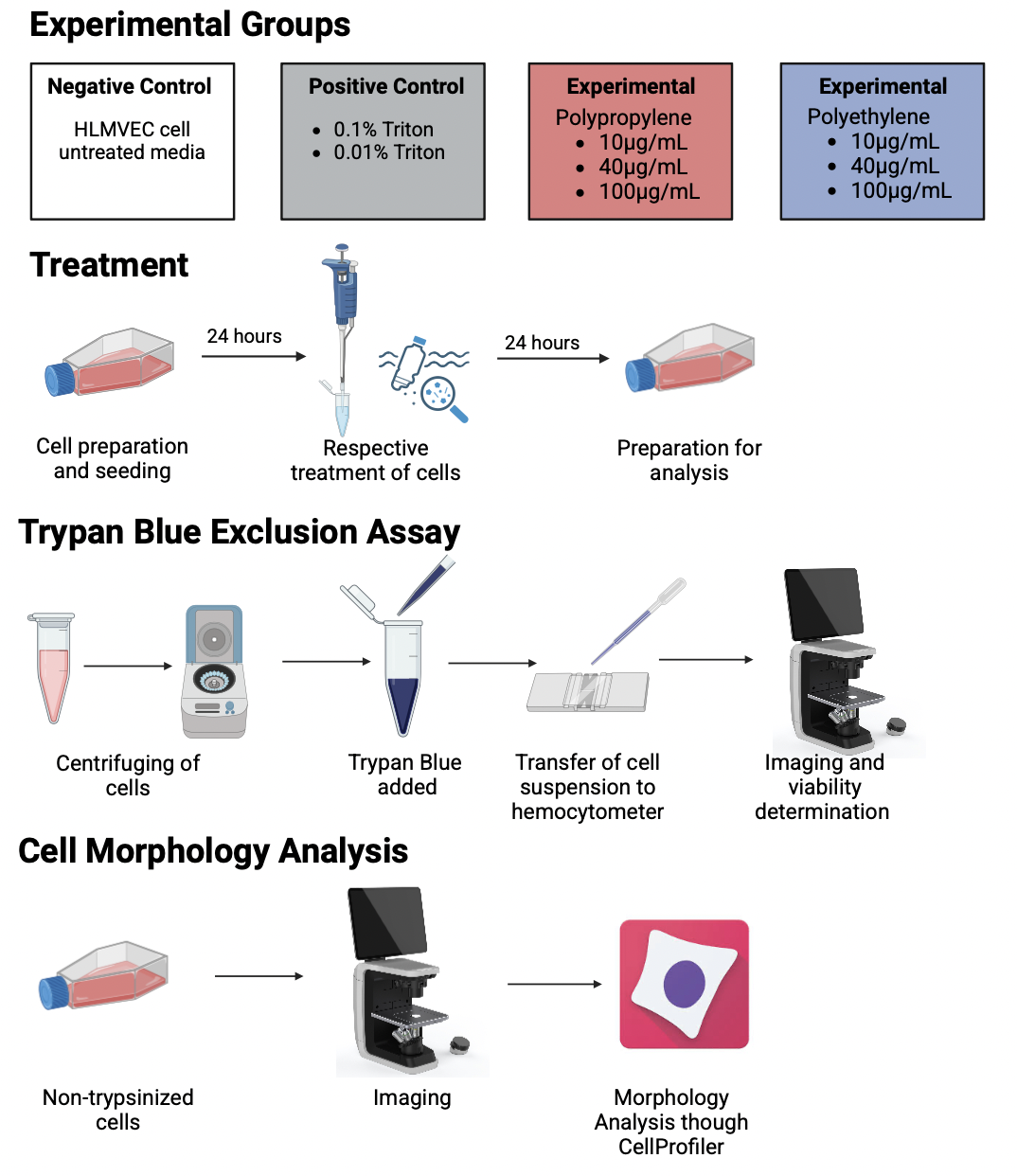
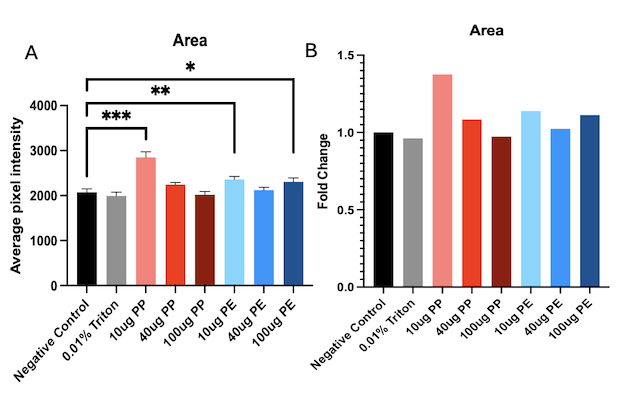
Microplastics are plastic particles that can range from 1 nanometer to 5 millimeters in size. These can be found everywhere: in the air we breathe to the water we drink. Even though they are so prevalent, microplastics were first discovered in 2004 by Marine Biologist Richard Thompson (Schmid et al., 2021). However, microplastics weren’t detected in the human body until 2022 when researchers in the Netherlands found microplastics in human blood samples (Roeloffs, 2024). Microplastics can be made of various materials and even though little is known about them, existing research about them shows their harmful effects on other living organisms. This raises the issue of the lack of knowledge regarding their effect on humans and thus the dangers that they are constantly exposed to.
Types of Microplastics
There are two main categories of microplastics: Primary and Secondary, which are differentiated by how they were created. Primary microplastics are produced by manufacturers to be small. They can come in the form of either pellets (called nurdles) or in the form of microbeads, which are often manufactured for the cosmetic and pharmaceutical industry (Microplastics – Pollution Tracker, 2016). Secondary microplastics, on the other hand, result from the breakdown of larger plastics – often beach litter or laundry (Microplastics – Pollution Tracker, 2016). Overall, there are 13 types of microplastics. However, the most common types of microplastics are polyethylene, polypropylene, and polystyrene (Olabode & Fulmer, n.d.).
Effects of Microplastics
Microplastics are a new discovery, and thus little research has been done on them. However, studies have shown that microplastics are extremely toxic, inducing oxidative damage, DNA damage, organ dysfunction, metabolic disorders, weakened immune responses, neurotoxicity, reproductive toxicity, and developmental toxicity (Li et al., 2023). However, most of the studies done have focused on organs directly exposed to microplastics, such as the lungs and small intestines. Minimal studies have been done on indirectly exposed organs, such as the heart, and correlating parts, such as blood vessels.
Endothelial Cells
Endothelial cells line all blood and lymphatic vessels found in the human body and thus play a key role in blood transport and human survival. In fact, these cells are often the first line of defense for any ingested toxin prior to entering the body. Endothelial cells are found most commonly in arteries, veins, capillaries, and lymph capillaries. In lymph capillaries, endothelial cells function as a semi-permeable barrier, allowing for the transport for lymph fluid along with facilitating the movement of immune cells to lymph nodes – which is necessary for the immune system to function (Cleveland Clinic, 2022). In arteries, veins, and capillaries, endothelial cells are responsible for producing glycocalyx, a vital substance for human survival (Villalba et al., 2021).
Vascular Endothelial Glycocalyx
The endothelial glycocalyx is a carbohydrate-rich, fibrous layer that lines the luminal surface of endothelial cells and blood vessels, thus playing many vital roles in human survival (Reitsma et al., 2007). One of the functions of the glycocalyx is permeability. The glycocalyx regulates transport of water proteins, and other essential molecules from the blood to the outside of blood vessels. Along with this, it can restrict certain molecules from passing through endothelial cells, thus protecting blood vessels. This restriction can be attributed to the structure and negative charge of the glycocalyx (Jin et al., 2021).The glycocalyx is also necessary for inflammation. It plays an essential role in the occurrence and development of inflammations, which is a defense mechanism that allows the body to fight off infections and heal. When inflammation occurs, the glycocalyx is shed, which makes it easier for leukocytes to bind to endothelial cells. This binding is essential for immune response. The glycocalyx plays a key role in the anticoagulant process as well. Under certain conditions, the interaction between endothelial cells and blood cells can be altered, avoiding thrombosis. By interacting with antithrombin III, thrombomodulin, tissue factor pathway inhibitors, and other molecules, the glycocalyx can induce anticoagulant effects (Jin et al., 2021). The glycocalyx is also necessary for sending signals. It detects changes in blood flow and then transmits that information to the endothelial cells. This enables the cells to respond with various morphological responses. The glycocalyx can also regulate apoptosis of the endothelial cells, allowing them to die whenever necessary and keeping them alive otherwise. A key function of the glycocalyx is cerebrovascular micro-homeostasis. The glycocalyx is responsible for maintaining the barrier function of the cerebral blood vessels. It regulates the permeability of the blood-brain barrier (BBB) and plays a key role in cerebrovascular coagulation and neuroinflammatory processes (Jin et al., 2021).