STEM I
Course Description
STEM is taught by Dr. Crowthers. In this class, we conduct a six-month-long major independent research project in a field of our choice.
We also get experience with technical writing, a small engineering project ("Build Something"), and effectively reading scientific articles.
Computationally Modeling the Ability of Cyclodextrins to Bind Short-Chain PFAS
Project Overview
The overall aim of this project was to find a solution to short-chain PFAS water contamination by
using computational chemistry to simulate the binding of various PFAS to different types of
cyclodextrins. The results of the study showed that beta-cyclodextrin with attached quaternary nitrogens
were most likely to bind to a variety of PFAS and are a good candidate for further research in the field.
Abstract
This project aims to use cyclodextrin compounds to adsorb per- and polyfluoroalkyl substances (PFAS) from water. PFAS are a growing problem in the modern environment. PFAS have a negatively charged head and a hydrophobic fluorinated tail. Current remediation strategies include adsorption with activated carbon and biochar. These work acceptably for PFAS with longer tails (long-chain or legacy PFAS), but legacy PFAS are being phased out for PFAS with shorter tails (short-chain PFAS). Current removal solutions rely on hydrophobic interactions and do not work for short-chain PFAS, which are less hydrophobic due to the shorter length of the hydrophobic part (the tail). In this project, we used computational chemistry to assess cyclodextrins as a remediation strategy for short-chain PFAS. Cyclodextrins are hollow truncated cone-shaped compounds that have a hydrophobic interior surface and hydroxyl groups along the edge which are capable of forming hydrogen bonds with another compound. These hydrogen bonds formed with the head of PFAS can compensate for the weaker hydrophobic interactions between short-chain PFAS tails and the interior surface. When docking the natural cyclodextrins to PFAS in AutoDock Vina, it was found that beta-cyclodextrins and gamma-cyclodextrins were significantly more effective than alpha-cyclodextrins at the a = 0.01 significance level. Additionally, several modified cyclodextrins were assessed with regards to their ability to bind PFAS. The results of this project can be used to create a more effective way to adsorb PFAS and a solution for the millions of people whose water is contaminated with these toxic chemicals.
Keywords: PFAS (per- and polyfluorinated alkyl substances), cyclodextrins, Sugammadex, water remediation, water contamination, computational chemistry, molecular docking
Graphical Abstract
Research Proposal
Please click HERE to visit my Research Proposal page with supporting documentation.
Researchable Question
What modifications can be made to a cyclodextrin compound so that it effectively binds PFAS?
Hypothesis
Beta-cyclodextrins with attached cationic residues will bind short-chain PFAS better than alpha-cyclodextrins, gamma-cyclodextrins, and beta-cyclodextrins without modification.
Background
Per- and polyfluoroalkyl substances, or PFAS, are potentially carcinogenic and endocrine-disrupting chemicals that have a widespread effect on natural water sources. Additionally, these substances are widespread and do not break down significantly once released, which leads to their eventual bioaccumulation, or environmental persistence caused by a buildup in the food chain (Dickman & Aga, 2022).
PFAS consist of a negatively-charged head group and a hydrophobic fluorinated tail. Legacy or long-chain PFAS, such as PFOS and PFOA, have longer hydrophobic tails. This means that several promising solutions for their cleanup have been proposed, such as physical adsorption by various substances. However, due to discoveries of the toxicity and carcinogenicity of legacy PFAS, the chemical industry has shifted away from the use of legacy PFAS to that of modern short-chain PFAS. These were initially thought to be less toxic than legacy PFAS; however, it has been suggested that short-chain PFAS may be similarly toxic to their longer counterparts and may persist longer in the environment. In addition, short-chain PFAS are more hydrophilic than legacy PFAS due to the shorter length of the hydrophobic tail part, which lowers the efficacy of the hydrophobic interactions adsorption mechanism commonly utilized for legacy PFAS cleanup (Li et al., 2020). Therefore, it is clear that short-chain PFAS should be given a high level of priority when deciding areas for further research surrounding PFAS remediation.
Currently, one of the most promising remediation methods for PFAS of either chain length is the usage of various adsorbents. Granular activated carbon (GAC) is a widely used adsorbent for long-chain PFAS, but is often affected by natural organic materials existing in the environment and does not effectively adsorb short-chain PFAS. In addition, this material can be more costly than some methods. The indicated adsorption mechanisms were primarily hydrophobic and electrostatic interactions (Dickman & Aga, 2022).
Due to these problems, biochars and modified biochars have been proposed as a more effective solution to the problem of PFAS contamination. Biochar is a carbon-based adsorbent derived from pyrolyzed natural biomass and has been shown to have better results than the leading solutions of GAC in some studies (Militao et al., 2023). As such, it is a cost-effective method for the removal of PFAS.
One study using biochar-impregnated alginate beads found that beads with a certain biochar content had up to 99% removal efficiency for PFOS, a type of legacy PFAS, at a starting PFOS concentration of 100 μg L−1. For PFBS, one of the newer short-chain PFAS, using similar beads in which the biochar had been modified with diammonium phosphate prior to its addition yielded a removal efficiency of 39.3%. This was comparable to commercial GAC. Therefore, the biochars used in this study proved more effective towards legacy PFAS, but showed some promise toward future applications for short-chain PFAS (Militao et al., 2023).
Another study investigated the effects of doping reed straw-derived biochar with iron and copper on its adsorption capacity for PFBA and PFPeA, two short-chain PFAS. Both types of doped biochar had higher adsorption capacities for both PFAS, showing some promise to this method (Liu et al., 2024). However, better short-chain solutions are still necessary, as the level of effectiveness still did not approach the level achieved for long-chain PFAS.
One new approach to this problem is the use of cyclodextrins. Cyclodextrins consist of six (alpha-cyclodextrin), seven (beta-cyclodextrin), or eight (gamma-cyclodextrin) glucose units arranged in a ring structure. This results in a unique truncated cone shape, where the hydroxyl groups along the smaller edge form hydrogen bonds with a molecule and the inside of the cone is capable of hydrophobic interactions (Esteso & Romero, 2024). Cyclodextrins, specifically the beta-cyclodextrin variety, have been proposed as a novel solution to the PFAS issue.
One study tested the use of beta-cyclodextrins with an attached positively-charged functional group to bind various short-chain PFAS. It was found that PFAS thread through the ring cavity of the cyclodextrin. The tail will have hydrophobic interactions with the hydrophobic cavity, and the head will have hydrogen bonding interactions with the hydroxyl groups on the smaller edge of the cyclodextrin. Although hydrophobic interactions are weaker in short-chain PFAS than in legacy PFAS, the electrostatic interactions from the attached functional group were comparable to adding another hydrogen bond. Therefore, it appears that short-chain PFAS removal by cyclodextrins should be further investigated (Weiss-Errico & O’Shea, 2019).
Due to the limited research involving cyclodextrins and short-chain PFAS, the molecular docking software AutoDock Vina was employed to simulate the binding of the natural and modified cyclodextrins to a selection of short-and long-chain PFAS.
Procedure
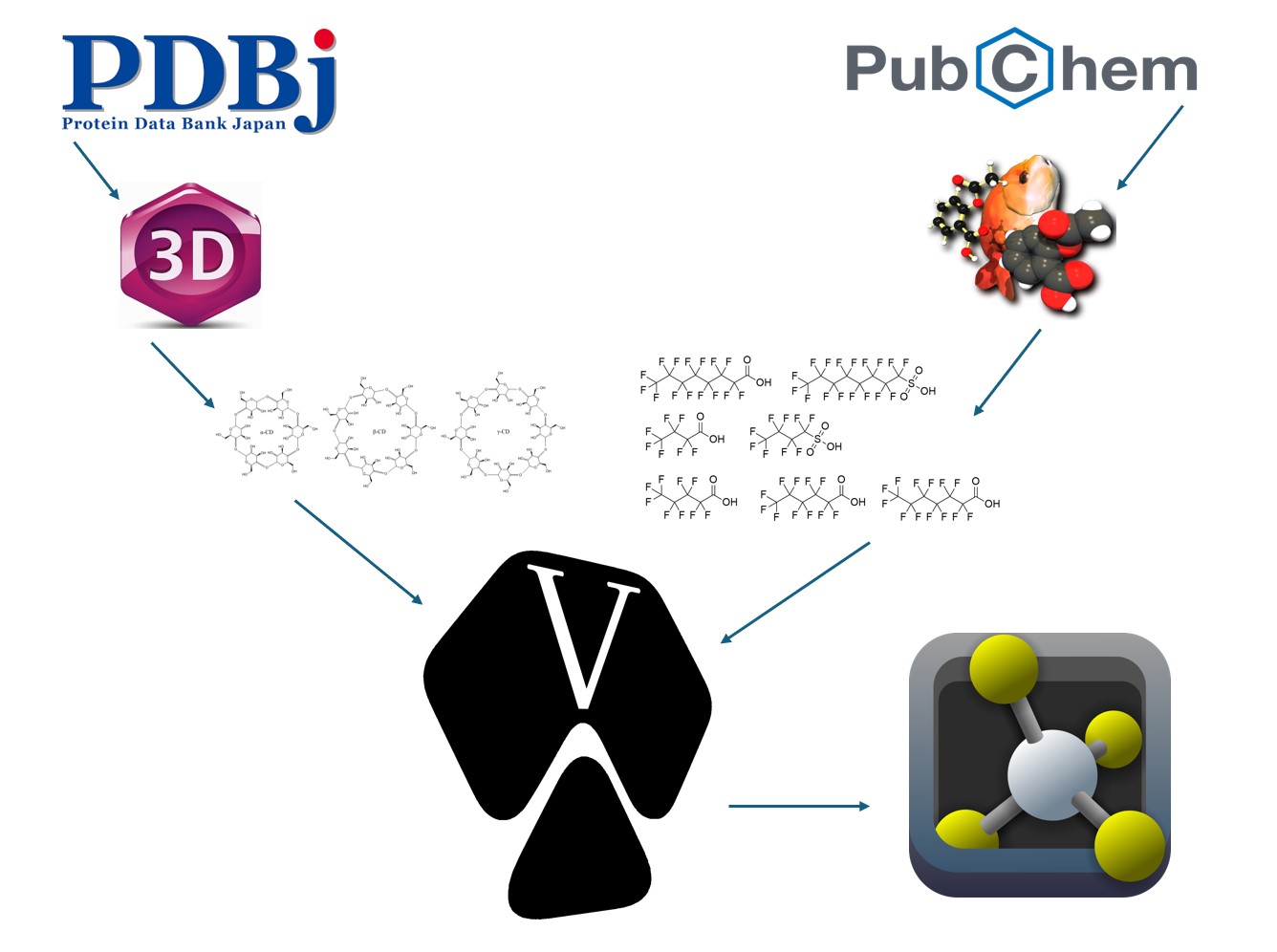
Equipment and Materials
The Protein Data Bank Japan was used to acquire .pdb 3D structural files of unmodified alpha-cyclodextrin, beta-cyclodextrin, and gamma-cyclodextrin. PubChem was used to acquire .xml 3D structural files of PFOA, PFOS, PFBA, PFBS, PFPeA, PFHpA, and PFHxA. Other software used included OpenBabel, ChemDraw and Chem3D, AutoDockTools, AutoDock Vina, and PyMOL. Data was analyzed with Microsoft Excel and a standard TI-83 Plus graphing calculator.
Technique 1 – Preparation of Natural Cyclodextrins
The .xml files of the 3D PFAS chemical structures were first converted to .pdb format using the conversion software OpenBabel. Next, the “grid box,” or space in which to model binding, was generated for each cyclodextrin in AutoDockTools and its dimensions in the x-, y-, and z- axes recorded in a configuration file. Each cyclodextrin and PFAS .pdb file was converted to a .pdbqt file with AutoDockTools.
Technique 2 – Docking of Natural Cyclodextrins
For each of the three cyclodextrins, the two long-chain and five short-chain PFAS were docked with it using AutoDock Vina. The top docking score for each PFAS-cyclodextrin combination was recorded in an Excel sheet and the .pdbqt file with the nine most likely poses saved locally. PyMOL was used to observe the likely interactions in each pose with each candidate PFAS for each cyclodextrin.
Technique 3 – Preparation of Modified Cyclodextrins
The first modification, which was obtained by replacing the hydroxyl groups on the shorter rim of gamma-cylodextrin was carried out by the mentor. The grid box and .pdbqt file were generated with AutoDockTools as previously described. The second modified cyclodextrin was developed by the student in Chem3D by replacing the hydroxyl groups in gamma-cyclodextrin with a nitrogen atom bonded to three methyl groups in addition to its link to the main molecule. Giving the nitrogen four bonds to carbon results in a permanent positive charge on the nitrogen. An energy minimization was performed in Chem3D to generate the most likely structure and bond angles. The grid box and .pdbqt file were generated with AutoDockTools.
Technique 4 – Docking of Modified Cyclodextrins
As described before, the modified cyclodextrins were docked to the PFAS sample in AutoDock Vina. PyMOL was used to observe the likely interactions in each pose.
Statistical Tests
A matched-pairs t-test was utilized to analyze the average difference in docking score for each pair of cyclodextrins for the seven-PFAS sample. The reason that this test was preferred over a two-sample t-test was that the same sample of PFAS was used for all cyclodextrins, so it is most accurate to compare the values found for each individual in the sample.
Figures
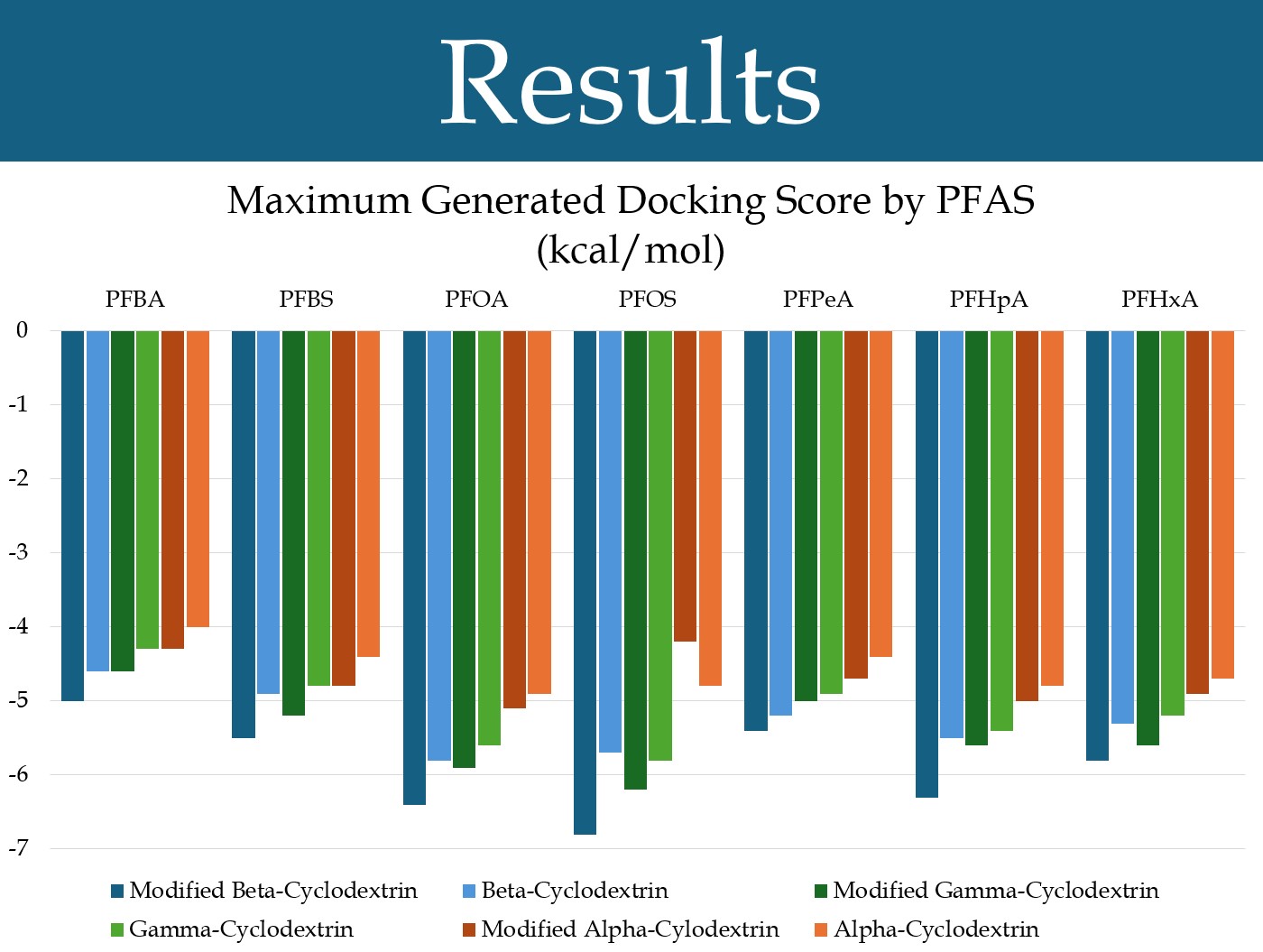
Figure 1: The docking scores for the most favorable pose generated with each cyclodextrin (grouped by PFAS).
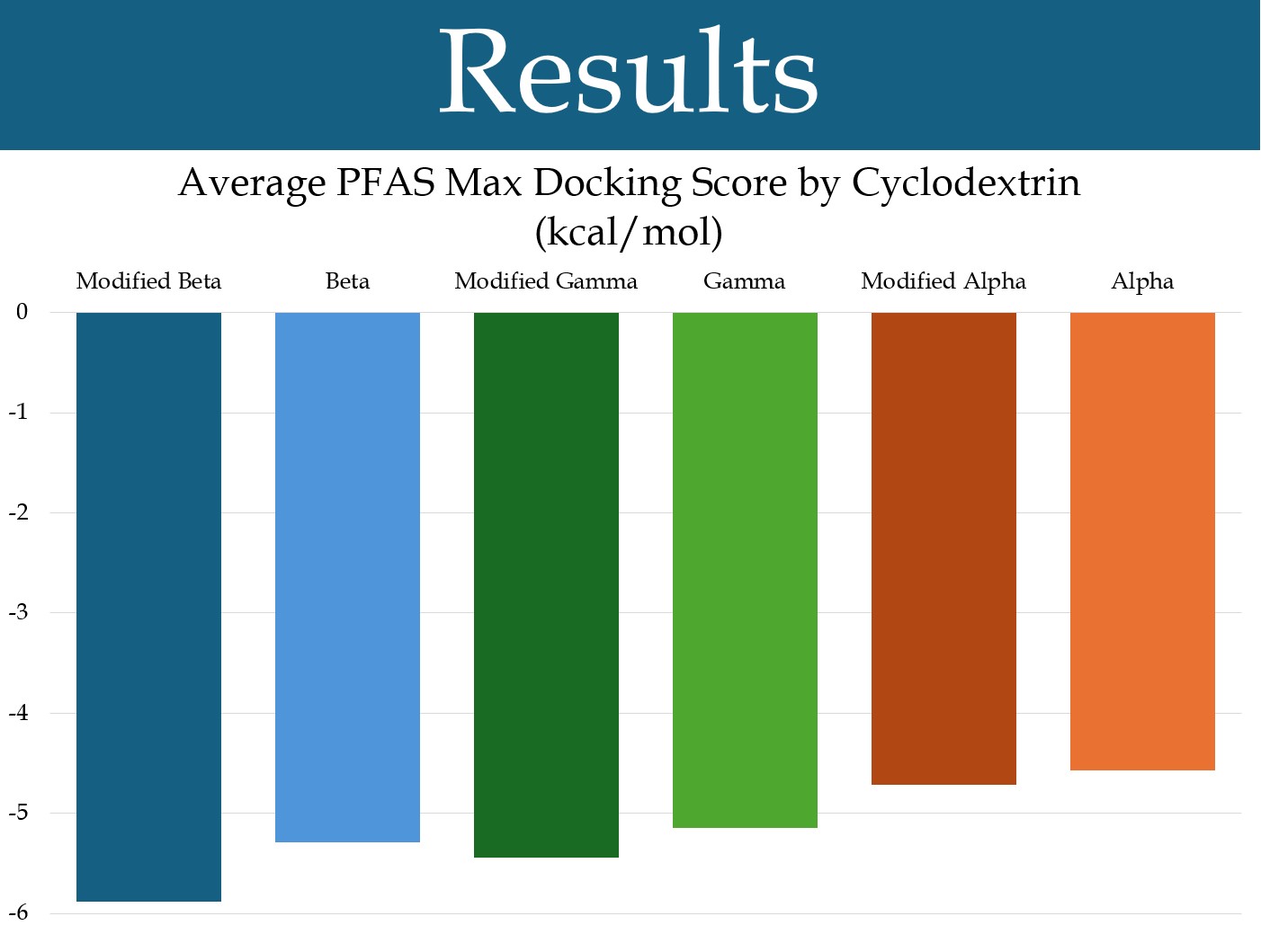
Figure 2: The average (by cyclodextrin) of the maximum docking scores for each PFAS in the sample.
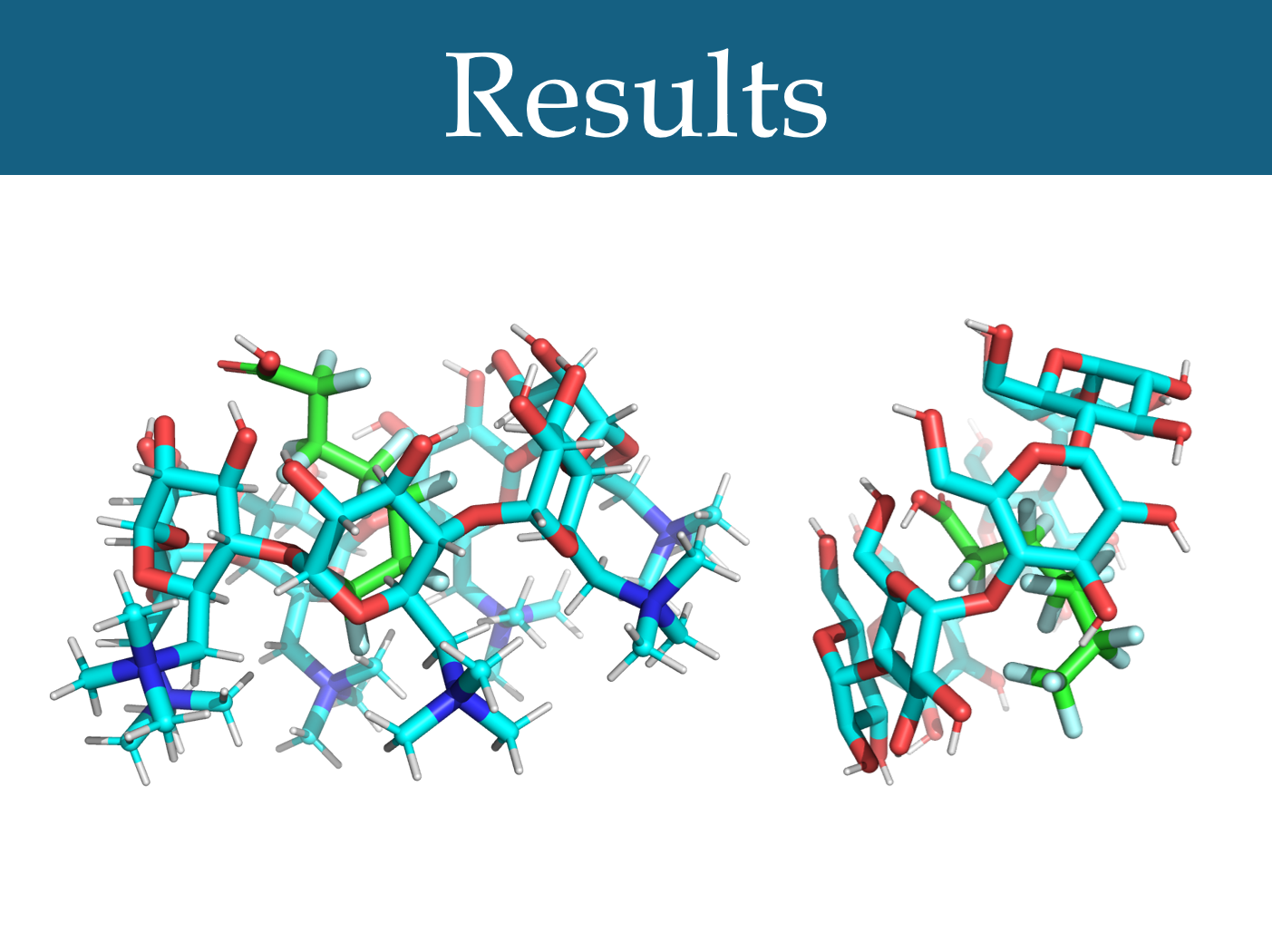
Figure 3: The optimal docking poses for modified β-CD and PFHpA (-6.3 kcal/mol) (left) and unmodified β-CD and PFHpA (-5.5 kcal/mol) (right)
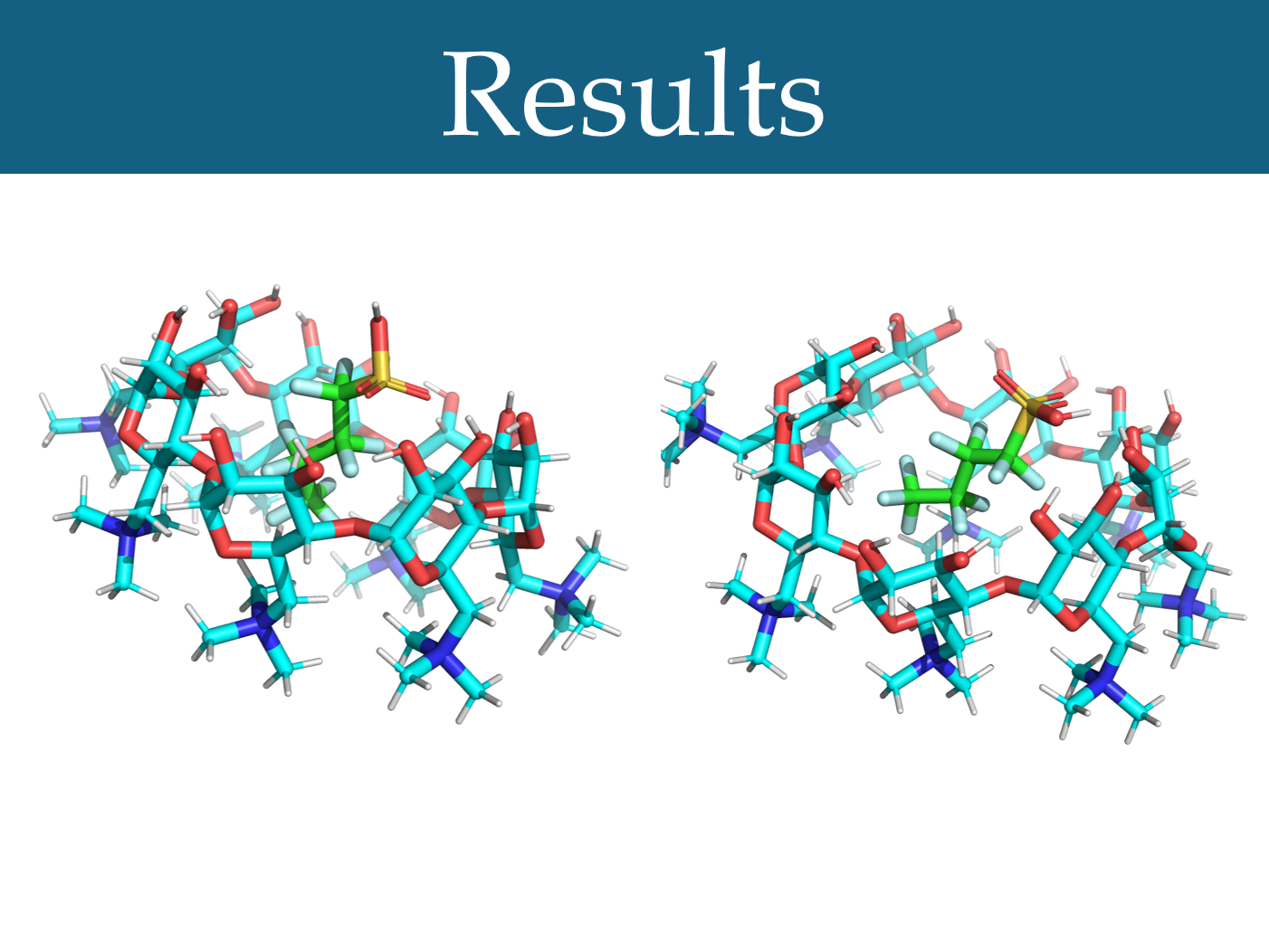
Figure 4: The optimal docking poses for modified β-CD and PFBS (-5.5 kcal/mol) (left) and modified γ-CD and PFBS (-4.9 kcal/mol) (right)
Analysis
Natural Cyclodextrins
Beta-Cyclodextrin
When the docking simulation was run, beta-cyclodextrin showed some results in binding PFAS. Although PFOA and PFOS, the two long-chain PFAS, showed the greatest degree of binding (as expected), promising scores were also obtained for short-chain PFAS (Figure 1).
Alpha-Cyclodextrin and Gamma-Cyclodextrin
For nearly all PFAS tested, including two common long-chain PFAS and five common short-chain PFAS, beta-cyclodextrin had the highest docking score, closely followed by gamma-cyclodextrin (Figure 2). Alpha-cyclodextrin was not as high. The average maximum docking score for each cyclodextrin is shown in the graph (Figure 2). In a matched-pairs t-test, beta-cyclodextrin was shown to have a significantly different average than alpha-cyclodextrin (***p<0.001), but had a lesser difference with gamma-cyclodextrin (*p<0.05). Gamma-cyclodextrin also had a significantly different average (***p<0.001) than alpha-cyclodextrin.
Modified Cyclodextrins
Hydroxyls Replaced With Amines
It was found that an amine-modified beta-cyclodextrin had significantly lower docking scores on average than regular beta-cyclodextrin, utilizing a matched-pairs t-test (***p<0.001).
Hydroxyls Replaced With Quaternary Nitrogens
Beta-cyclodextrins modified by replacing their primary hydroxyls with quaternary nitrogens were found to have a significantly higher average docking score than unmodified beta-cyclodextrins, using a matched-pairs t-test (***p<0.001) (Figure 3). Gamma-cyclodextrins with this modification also had a significantly higher average docking score than unmodified gamma-cyclodextrins (***p<0.001) (Figure 3). Alpha-cyclodextrins with this modification did not have a significantly different docking score, on average, than unmodified alpha-cyclodextrins (p = 0.152) (Figure 3). Additionally, it was found that beta-cyclodextrins modified in this way had a significantly higher average docking score than gamma-cyclodextrins with this modification (***p<0.001).
Discussion & Conclusion
It was found that beta-cyclodextrins with attached cationic residues will bind short-chain PFAS better than alpha-cyclodextrins, gamma-cyclodextrins, and beta-cyclodextrins without modification. Out of the natural cyclodextrins, beta-cyclodextrin demonstrated a greater ability to bind PFAS than gamma-cyclodextrin (*p<0.05) and alpha-cyclodextrin (***p<0.001). When modified by replacing the primary terminal hydroxyl groups with quaternary nitrogens (permanently positively charged nitrogen atoms with three additional methyl groups), beta-cyclodextrin demonstrated a greater ability to bind PFAS than without the modification (***p<0.001). Additionally, beta-cyclodextrin with the modification demonstrated a greater ability to bind PFAS than gamma-cyclodextrin with the modification (***p<0.001). This confirms the hypothesis that beta-cyclodextrins with positive groups will bind PFAS better than unmodified cyclodextrins.
One potential limitation for this project is the use of AutoDock Vina, which outputs a “docking score” which cannot directly be compared to binding energies derived from other methods due to certain inaccuracies in the scoring function. However, for the scope of this project, it could still be assumed that the energies obtained represented relative abilities to bind each PFAS. One challenge addressed was the failure of the primary modification to perform better than the control or unmodified cyclodextrin. However, another modification was developed that would result in a permanent positive charge on each nitrogen atom, which succeeded. Other challenges addressed included problems getting the AutoDockTools program to properly generate the .pdbqt files, as first attempts resulted in Python errors and/or files that could not be read by the Vina program. However, through background research, an alternative method was developed to generate .pdbqt files for the macromolecule (cyclodextrins).
For this project, matched-pairs t-testing was continuously used due to the fact that each cyclodextrin was tested with the same sample of two long-chain PFAS and five short-chain PFAS. The reason that long-chain PFAS were included in this study as well as a variety of chain lengths for the short-chain PFAS is that potential modifications would be expected to bind both types of PFAS, so although the primary goal of this research is to address short-chain PFAS, it is also important to consider long-chain PFAS, as PFOA and PFOS are currently some of the most widespread PFAS in the environment.
This work expands on existing gaps in the research regarding ways to bind short-chain PFAS. Short-chain PFAS currently do not have adsorbents as effective as those for long-chain PFAS (Li et al., 2020; Militao et al., 2023). Therefore, some research has focused on looking at cyclodextrins as a potential solution (Abaie et al., 2024). Preliminary work has yielded promising results, such as one study which found positively charged polymer beta-cyclodextrin adsorbents up to 20 times as effective for certain short-chain PFAS (Weiss-Errico & O’Shea, 2019). This research used computational chemistry to easily analyze a wider variety of adsorbents and PFAS, focusing on opening paths for future synthesis. This paper suggests that quaternary nitrogens and positively-charged derivatives of beta-cyclodextrin should be further explored as a potential method for short-chain PFAS adsorption.
Future research would involve utilizing different substitutions of positive functional groups on the cyclodextrin so as to find the best way to bind short-chain PFAS. Additionally, molecular modeling techniques could be used to determine what types of forces are playing a role in the binding. Finally, wet-bench experiments could confirm these results and provide an overall solution.
The objective of this study was to develop a more effective compound to bind short-chain PFAS and remove them from water, since these chemicals are often ignored in existing literature. To do this, the molecular docking program AutoDock Vina was employed to study unmodified cyclodextrins and cyclodextrins modified by replacing their terminal hydroxyl groups with quaternary nitrogens with regards to their ability to bind two long-chain PFAS and five short-chain PFAS. It was found that beta-cyclodextrin with modification was significantly better at binding PFAS (had higher docking scores) than any of the five other combinations of cyclodextrin size and modification vs. no modification.
References
- Abaie, E., Kumar, M., Kumar, N., Sun, Y., Guelfo, J., Shen, Y., & Reible, D. (2024). Application of β-Cyclodextrin Adsorbents in the Removal of Mixed Per- and Polyfluoroalkyl Substances. Toxics (Basel), 12(4), 264. https://doi.org/10.3390/toxics12040264
- Anderson, A., García-Fandiño, R., Piñeiro, Á., & O'Connor, M. S. (2024). Unraveling the molecular dynamics of sugammadex-rocuronium complexation: A blueprint for cyclodextrin drug design. Carbohydrate Polymers, 334, 122018. https://doi.org/https://doi.org/10.1016/j.carbpol.2024.122018
- Dickman, R. A., & Aga, D. S. (2022). A review of recent studies on toxicity, sequestration, and degradation of per- and polyfluoroalkyl substances (PFAS). Journal of Hazardous Materials, 436, 129120. https://doi.org/https://doi.org/10.1016/j.jhazmat.2022.129120
- Eberhardt, J., Santos-Martins, D., Tillack, A. F., & Forli, S. (2021). AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. Journal of Chemical Information and Modeling, 61(8), 3891-3898. https://doi.org/10.1021/acs.jcim.1c00203
- Esteso, M. A., & Romero, C. M. (2024). Cyclodextrins: Properties and Applications. Int J Mol Sci, 25(8). https://doi.org/10.3390/ijms25084547
- Fenton, S. E., Ducatman, A., Boobis, A., DeWitt, J. C., Lau, C., Ng, C., Smith, J. S., & Roberts, S. M. (2021). Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem, 40(3), 606-630. https://doi.org/10.1002/etc.4890
- Li, F., Duan, J., Tian, S., Ji, H., Zhu, Y., Wei, Z., & Zhao, D. (2020). Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chemical Engineering Journal, 380, 122506. https://doi.org/https://doi.org/10.1016/j.cej.2019.122506
- Liu, N., Li, Y., Zhang, M., Che, N., Song, X., Liu, Y., & Li, C. (2024). Efficient adsorption of short-chain perfluoroalkyl substances by pristine and Fe/Cu-loaded reed straw biochars. Science of The Total Environment, 946, 174223. https://doi.org/https://doi.org/10.1016/j.scitotenv.2024.174223
- Militao, I. M., Roddick, F., Fan, L., Zepeda, L. C., Parthasarathy, R., & Bergamasco, R. (2023). PFAS removal from water by adsorption with alginate-encapsulated plant albumin and rice straw-derived biochar. Journal of Water Process Engineering, 53, 103616. https://doi.org/https://doi.org/10.1016/j.jwpe.2023.103616
- Murray, C. C., Vatankhah, H., McDonough, C. A., Nickerson, A., Hedtke, T. T., Cath, T. Y., Higgins, C. P., & Bellona, C. L. (2019). Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. Journal of Hazardous Materials, 366, 160-168. https://doi.org/https://doi.org/10.1016/j.jhazmat.2018.11.050
- Nag, K., Singh, D. R., Shetti, A. N., Kumar, H., Sivashanmugam, T., & Parthasarathy, S. (2013). Sugammadex: A revolutionary drug in neuromuscular pharmacology. Anesth Essays Res, 7(3), 302-306. https://doi.org/10.4103/0259-1162.123211
- O'Boyle, N. M., Banck, M., James, C. A., Morley, C., Vandermeersch, T., & Hutchison, G. R. (2011). Open Babel: An open chemical toolbox. Journal of Cheminformatics, 3(1), 33. https://doi.org/10.1186/1758-2946-3-33
- Tokranov, A. K., Ransom, K. M., Bexfield, L. M., Lindsey, B. D., Watson, E., Dupuy, D. I., Stackelberg, P. E., Fram, M. S., Voss, S. A., Kingsbury, J. A., Jurgens, B. C., Smalling, K. L., & Bradley, P. M. (2024). Predictions of groundwater PFAS occurrence at drinking water supply depths in the United States. Science, 386(6723), 748-755. https://doi.org/doi:10.1126/science.ado6638
- Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455-461. https://doi.org/https://doi.org/10.1002/jcc.21334
- Weiss-Errico, M. J., & O’Shea, K. E. (2019). Enhanced host–guest complexation of short chain perfluoroalkyl substances with positively charged β-cyclodextrin derivatives. Journal of inclusion phenomena and macrocyclic chemistry, 95(1-2), 111-117. https://doi.org/10.1007/s10847-019-00930-w
February Fair Poster






