STEM I
STEM I takes up A, B, and about half of C Term.
It involves the Independent Research Project, in which students get to design and conduct a science/engineering/math research project based off of their personal interests.
A Term mostly entails brainstorming and research; B Term involves more research and the acquisition of preliminary data, culminating in the December Fair; C Term wraps up the project with February Fair.
From there, a selection of 12 students make it on to the Worcester Regional Science and Engineering Fair (WRSEF), and potentially onto larger conferences thereafter.
This page is currently under construction as this experiment progresses!
Methylene Blue as a Means to Bypass the Compromised Complex I in Mitochondrial Disease
In individuals affected by Complex I mitochondrial defects, sub-optimal rates ATP synthesis can cause a variety of physiological symptoms ranging from chronic fatigue to tissue necrosis.
This issue is exacerbated by the introduction of Complex I-inhibitory anesthetics.
My STEM I project focuses on using an alternative electron carrier, methylene blue, to divert electrons away from the damaged Complex I to allow the mitochondrial Electron Transport Chain to function normally, especially under anesthetic stress.
Scroll down this page to learn more about my study!
Abstract and Graphical Abstract
~Click here to view my full research proposal~
Background:

Mitochondrial Diseases
Mitochondrial diseases are a symptomatically diverse group of disorders characterized by mitochondrial dysfunction. The mitochondria are primarily responsible for energy production through oxidative phosphorylation and are instrumental to many of the metabolic pathways in the human body. Mitochondrial diseases are most commonly caused by genetic mutations in either mitochondrial DNA (mtDNA) or nuclear DNA (nDNA), consequently impairing the mitochondrial respiratory chain, which generates ATP (Gorman & Chinnery, 2016). Bodily functions, ranging from the conduction of nerve impulses to the regeneration of damaged tissue, all rely upon ATP, so it is understandable that a lack thereof would be detrimental to a patient’s health. Mitochondrial diseases affect virtually every tissue in the body, particularly attacking those with high energy demands, such as the brain, heart, and muscle (Sasano, 2007). Clinical manifestations vary greatly depending on the type and severity of the condition but can range from mild muscle weakness to neurodegeneration and multi-organ failure. Effective therapeutic strategies remain limited, highlighting the urgent need for new approaches to treatment and management despite advances in understanding the genetic and biochemical bases of these diseases (Gorman & Chinnery, 2016).
Complex I Inhibition & Anesthetics
Inhibition of Complex I of the Electron Transport Chain is a hallmark of several mitochondrial diseases. Complex I catalyzes the oxidation of NADH oxidoreductase, reducing ubiquinone to ubiquinol and generating a proton gradient across the inner mitochondrial membrane (Sharma, et al., 2009). This gradient allows protons to flow back through the mitochondrial matrix via ATP synthase, which provides the energy necessary to combine ADP and inorganic phosphate (Pi) into ATP in later complexes (Pereira, et al., 2023). Inhibition of Complex I not only compromises the rest of the process of mitochondrial respiration but enhances the generation of reactive oxygen species (ROS), known proponents of tissue damage (Sharma, 2007). Leaving Complex II as the only functional gateway to the rest of the Electron Transport Chain reduces the number of electrons eventually transported to Complex III.
Certain substances, such as rotenone and paraquat, can integrate easily with the mitochondrial membrane, granting them increased access to ubiquinone binding sites. These compounds interfere with electron transport by either directly blocking electron flow or prematurely diverting electrons, effectively truncating the pathway. As a result, oxidative phosphorylation is disrupted, directly leading to decreased ATP production. Additionally, since electrons are forced out of the Electron Transport Chain, they can interact prematurely with oxygen outside of the system and lead to an accumulation of reactive oxygen species (ROS). The buildup of ROS further contributes to cellular damage and oxidative stress, which are closely linked to various neurodegenerative disorders and other metabolic diseases. The presence of foreign bodies at this crucial point of the Electron Transport Chain has the potential to disrupt ATP production, leading to symptoms that mimic mitochondrial dysfunction, including fatigue, neurodegeneration, and metabolic imbalances (Pereira, 2023).
Popular general anesthetics such as isoflurane have been shown to inhibit Complex I in a similar manner, raising a concern about surgical practices with regard to mitochondrial disease patients (Vanlander, 2012). The anesthetic’s inhibitory properties may exacerbate existing symptoms in those with pre-existing mitochondrial dysfunction, leading to prolonged post-operation recovery time, worsened neurological symptoms, organ failure, and even sudden death. Understanding the interactions between anesthetic agents and the Electron Transport Chain is crucial for improving surgical protocol for mitochondrial disease patients (Sasano, 2007).
Anti-Inhibitory Properties of Methylene blue
Methylene blue (MB), a popular tissue stain, has been at the forefront of mitochondrial disease research in recent years. MB can interact with components of the mitochondrial electron transport chain by donating electrons; it has been shown to bypass Complex I by acting as an alternative electron carrier (Gureev, 2022). If Complex I was already damaged from a pre-existing disease, treatment with MB would be able to restore some degree of mitochondrial function (Lee & Boelsterli, 2014). This property renders MB a strong candidate for clinical application in mitochondrial diseases, and as a preventative measure for such patients exposed to Complex I inhibitory substances. Recent studies have also indicated that MB offers neuroprotective, cardioprotective, and cytoprotective benefits, making it even more appealing an option to treat mitochondrial disease, which so often damages the brain, heart, and cells due to their high energy demands (Lee & Boelsterli, 2014).
Study
If proven effective, MB therapy has the potential to offer a robust preventative measure for mitochondrial disease patients in the perioperative setting. This study aims to investigate the inhibitory properties of isoflurane on Complex I activity and the potential of MB as a therapeutic drug for Complex I-related mitochondrial diseases. Rotenone, which inhibits Complex I via the ubiquinone-binding sites, mimics the behavior of isoflurane in isolated C. elegans mitochondria, and was therefore a suitable candidate for the inhibitory treatment. For two treatment groups – with versus without MB – spectrophotometric analysis of enzyme absorption in Complex III was conducted. In Complex III, cytochrome c is reduced by electrons carried by ubiquinol. When electrons are successfully diverted from the compromised Complex I, Complex III activity in the MB group responds with increased activity compared to the non-MB group, as measured through reduced cytochrome c. Greater proportions of cytochrome c reductase in inhibited samples treated with MB were measured at 550 nm for maximized absorbance (Vanlander, A. V., et al., 2012). Furthermore, the enzymatic assay was performed on samples of mitochondria extracted from C. elegans mutated for mitochondrial myoencephalopathy, strain LB10, to provide more realistic insight into MB’s uses in the specific patient population. This run also yielded results corroborating the hypothesis that MB increases the flow of electrons Complex III when Complex I is unable to fulfill that function.
Full Procedure:
(As annotated on 11/07/2024 for the acquisition of preliminary data.)
Results
(As seen on the poster panels for February Fair.)
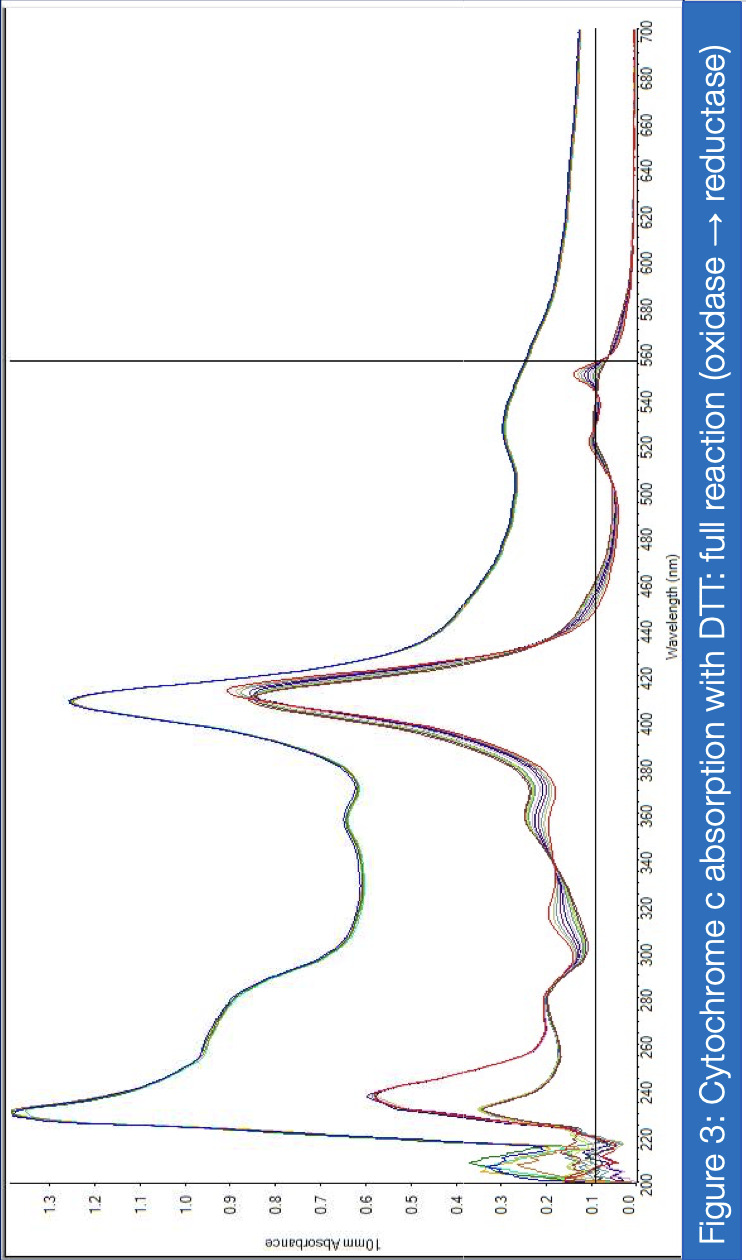
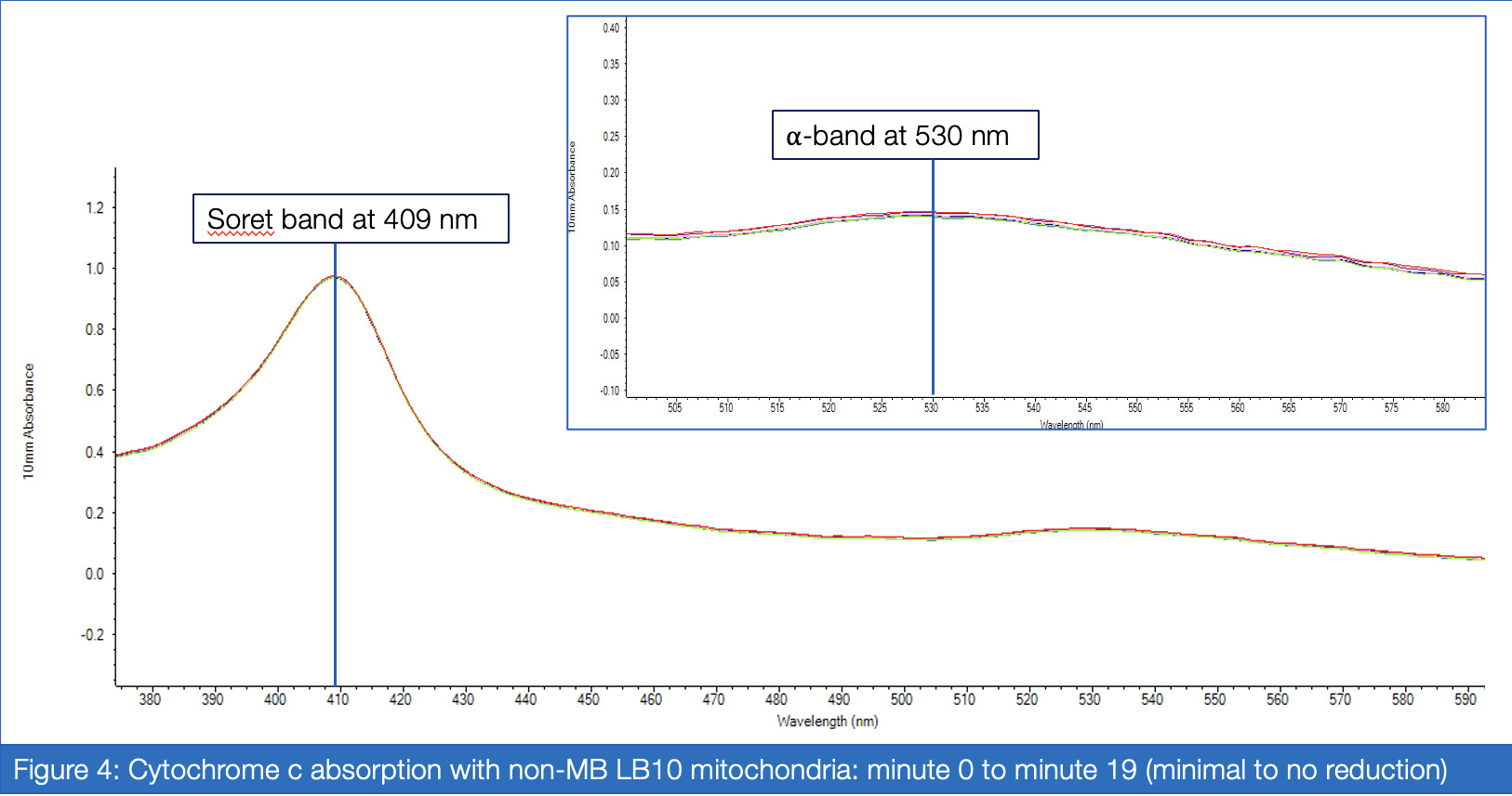
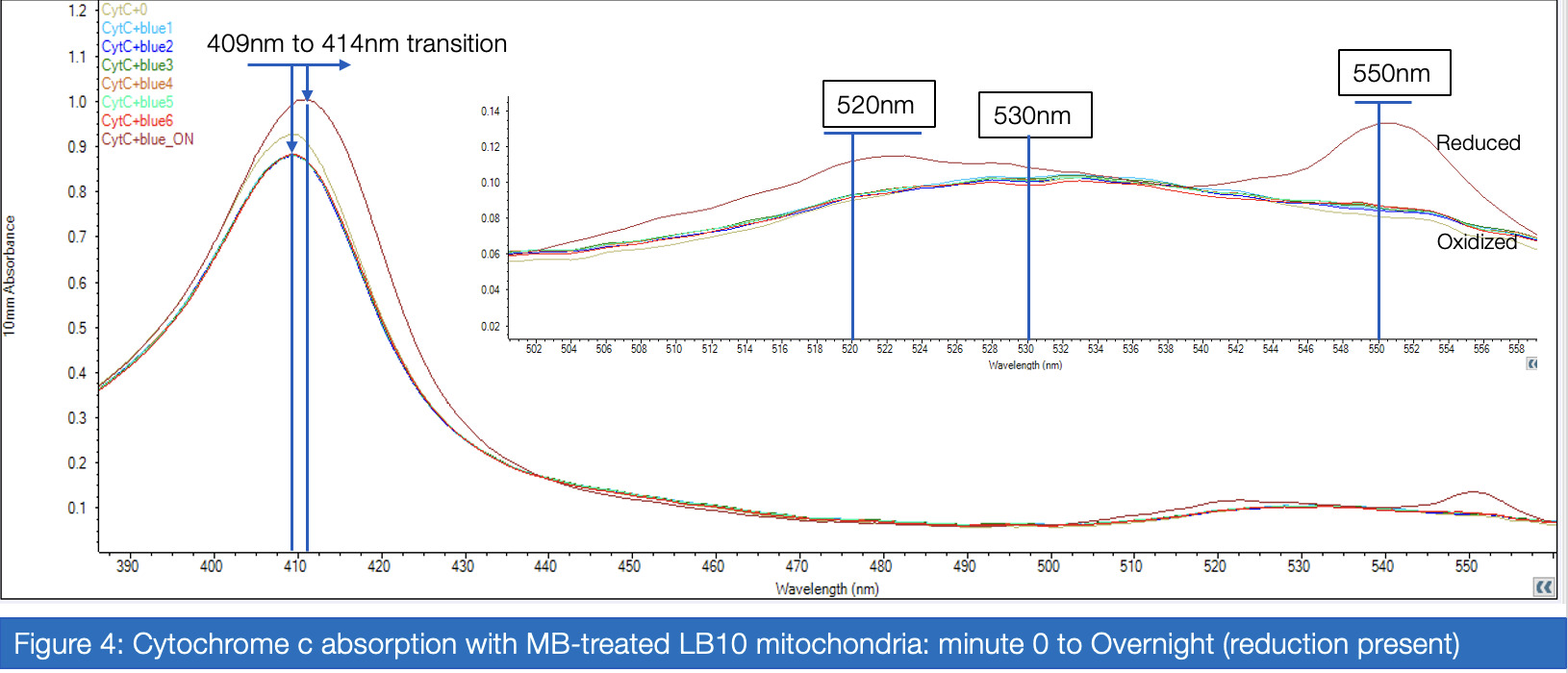
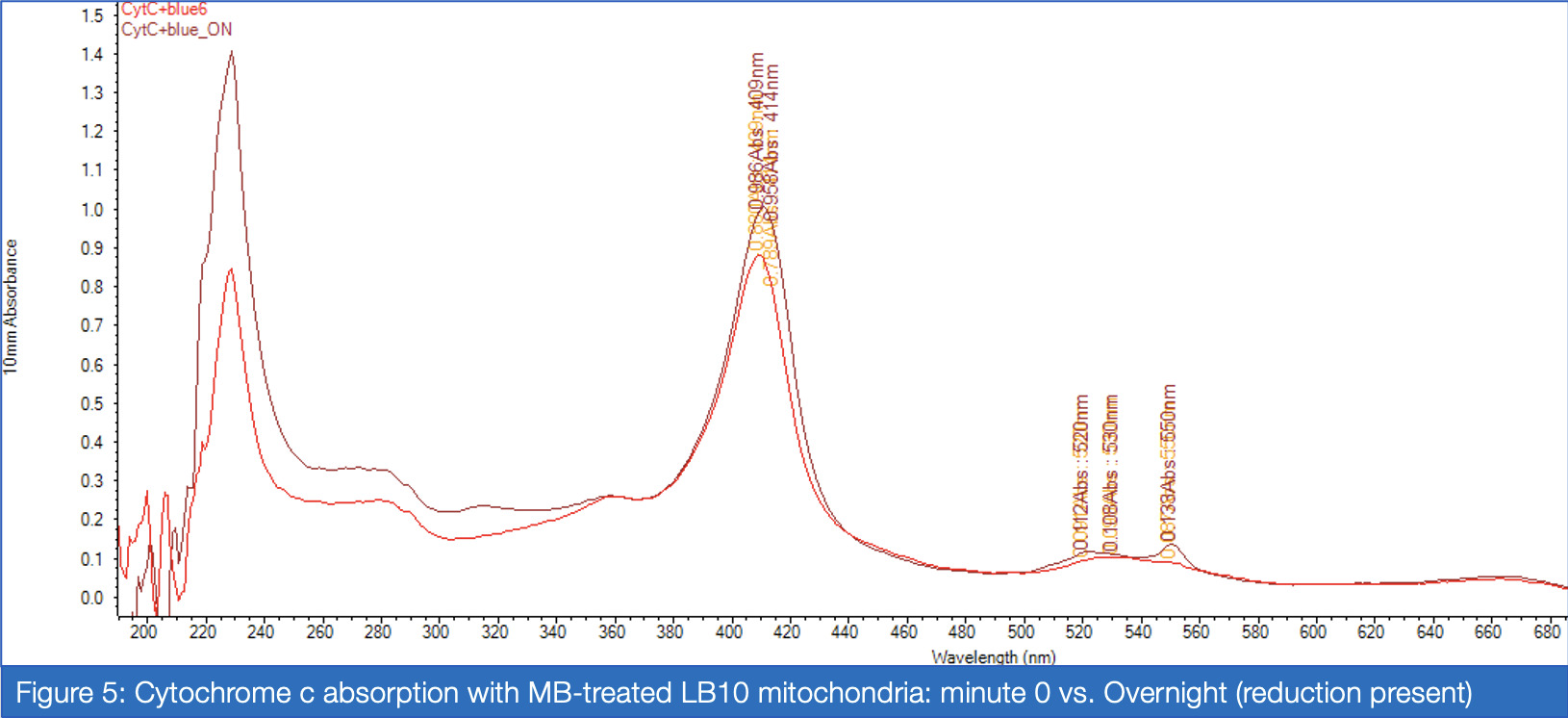
Analysis
[finish upon final data acquisition]

References
Poster Panels
(As used for February Fair, on 02/20/2025)




