Stem I
STEM with Science and Technical Writing I, taught by Dr. Crowthers, is Mass Academy’s
most "iconic" class, spanning August to March. In this class, we are not only exposed to science
literature but also write our own through an independent research project on a topic of our choosing. This section of the course concluded on February 20th, 2025
with the Mass Academy February Fair!
Using Neoblast Proliferation in Bioprinted Scaffolds as a Model for Metastasis
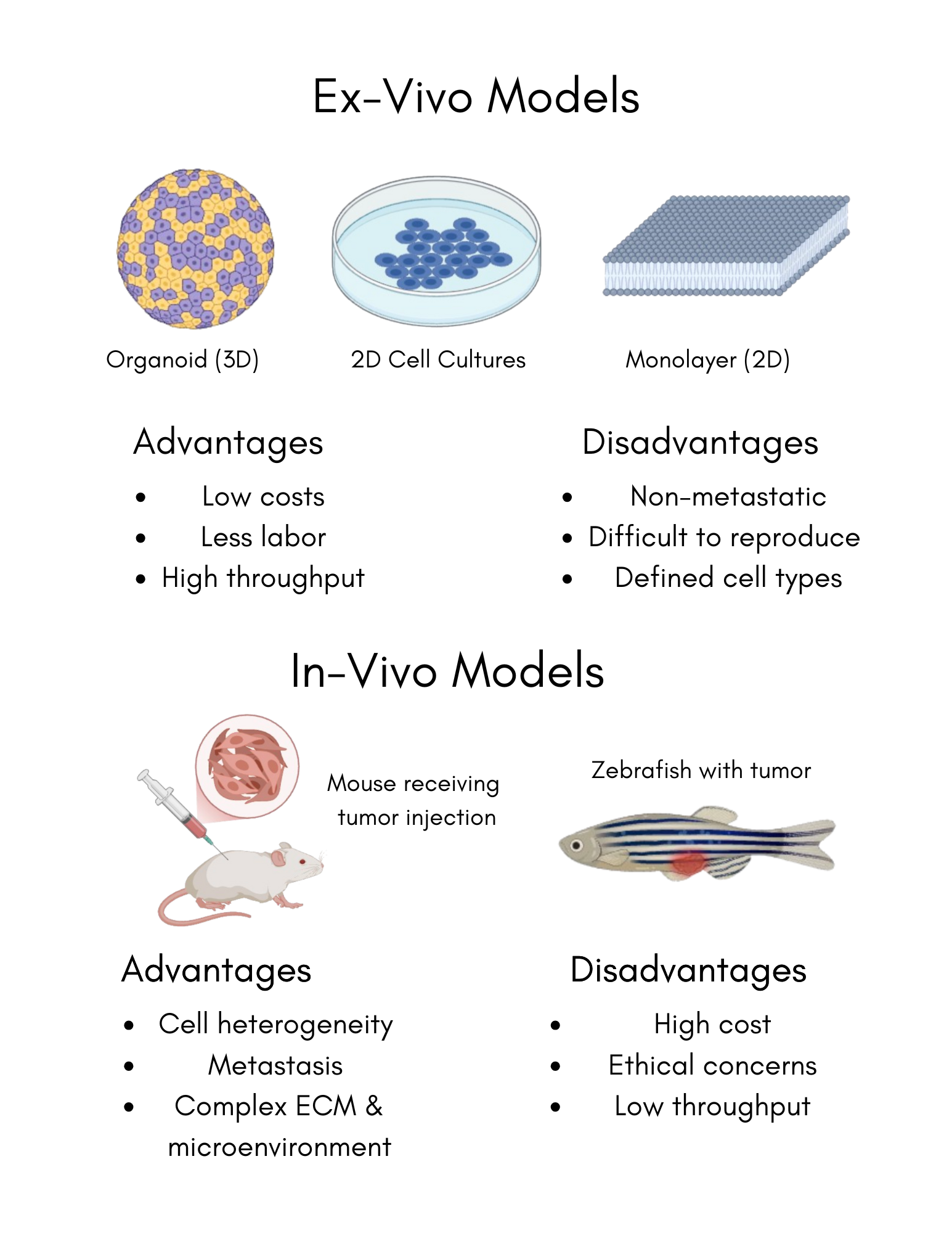
When considering the future of regenerative biology, bioprinting stands at the forefront due to its ability to manufacture biocompatible scaffolds that are organized and controllable. This innovative technology promotes cell growth in models that are more effective and viable than those currently used for studying cell proliferation and reproduction (Cellink, 2019). To understand biology, cell proliferation and reproduction must be studied as they provide the foundation for various biological processes, which significantly impact health, disease, and medicine. One of the most significant areas is cancer, where uncontrolled cell proliferation leads to tumor growth; unchecked cell growth is a root cause of tumor development and metastasis (Puls et al., 2018). As a result, modern research surrounding cancer to develop anticancer therapies requires a thorough comprehension of cell proliferation. Currently, models to study cell proliferation are produced in two ways: animal testing and 2D models. However, both methods have drawbacks which significantly impact their uses in a research environment. While models created through animal testing provide an accurate cell environment, it is expensive, time-consuming, and can potentially raise ethical concerns (Zhang et al., 2016). 2D models, such as traditional cell cultures grown in petri dishes, can cost significantly less but often has low reproducibility rates, suggesting that the alternative to animal testing is unable to properly showcase cell-to-cell and cell-to-environment complexities. Through bioprinting, these concerns can be mitigated while the benefits of each model are kept. Bioprinting allows for the facilitation of layer-by-layer scaffolds that loosely mimic native tissue architecture, offering a low cost, ethical, and highly reproducible approach by combining the precision of 3D printing with biological systems (Shukla et al., 2022). These scaffolds are a key to studying cancer cell proliferation as they facilitate an ideal environment for cellular behavior.
Abstract
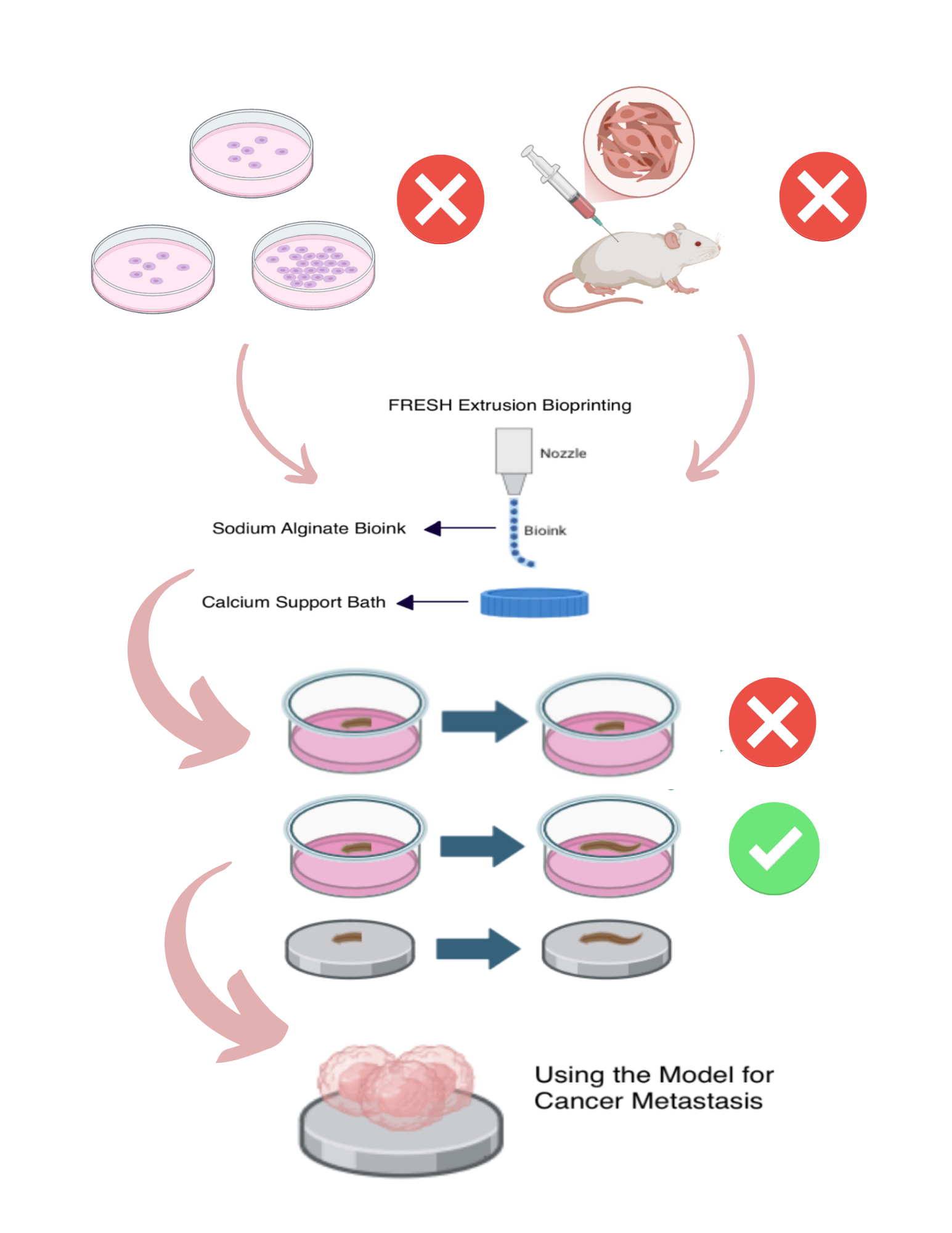
Cancer, characterized by the uncontrolled division of cells within the body, becomes particularly dangerous when it metastasizes, or spreads to other parts of the body. Among individuals with metastatic cancer, the median survival time is approximately 10 months, highlighting a critical need for improved research models. Current models for cancer metastasis, such as animal systems and 2D cell cultures, are both costly and challenging to reproduce, creating substantial barriers to cancer research and delaying the development of potentially life-saving therapies. Bioprinting technology offers a promising alternative to these models by enabling the creation of cost-effective, reproducible models for studying cancer metastasis, provided that a functional microenvironment can be established within the bioprinted scaffold. The aim of this project is to develop a cost-effective, reproducible bioprinted model to mimic the proliferating aspect of cancer metastasis. Known for their abundant neoblasts with a highly proliferative nature, planaria serve as a biological analogue for metastatic cancer cells due to their capacity for rapid cell proliferation and tissue regeneration. When the regeneration of planaria within the bioprinted model is compared to that in their natural environment, the results are expected to be consistent, demonstrating the feasibility of developing an initial model for cancer metastasis. Possible mechanisms to enhance the proliferation of planaria within the model to better replicate the behavior of aggressive cancers can be further explored by modifying parameters of the bioprinting process.
Researchable Question
Hypothesis
Can a cost-effective and replicable model for cancer cell metastasis be made using 3D bioprinting technology?
Under a compatible environment, the inherently cost-effective and replicable nature of bioprinting can
be merged with planarian neoblasts to create an accurate model to study cancer metastasis due to the similarity between neoblast
division to metastasis.
Background
-->
Procedure
-->
This project demonstrates the viability of bioprinted constructs as models of cancer metastasis and cell proliferation.
Current models for cancer metastasis, such as animal systems and 2D cell cultures, are both costly and challenging to reproduce,
creating substantial barriers to cancer research and delaying the development of potentially life-saving therapies. Therefore,
this researched aimed to bridge this lack of a reproducible, cost-effective model for metastasis research.
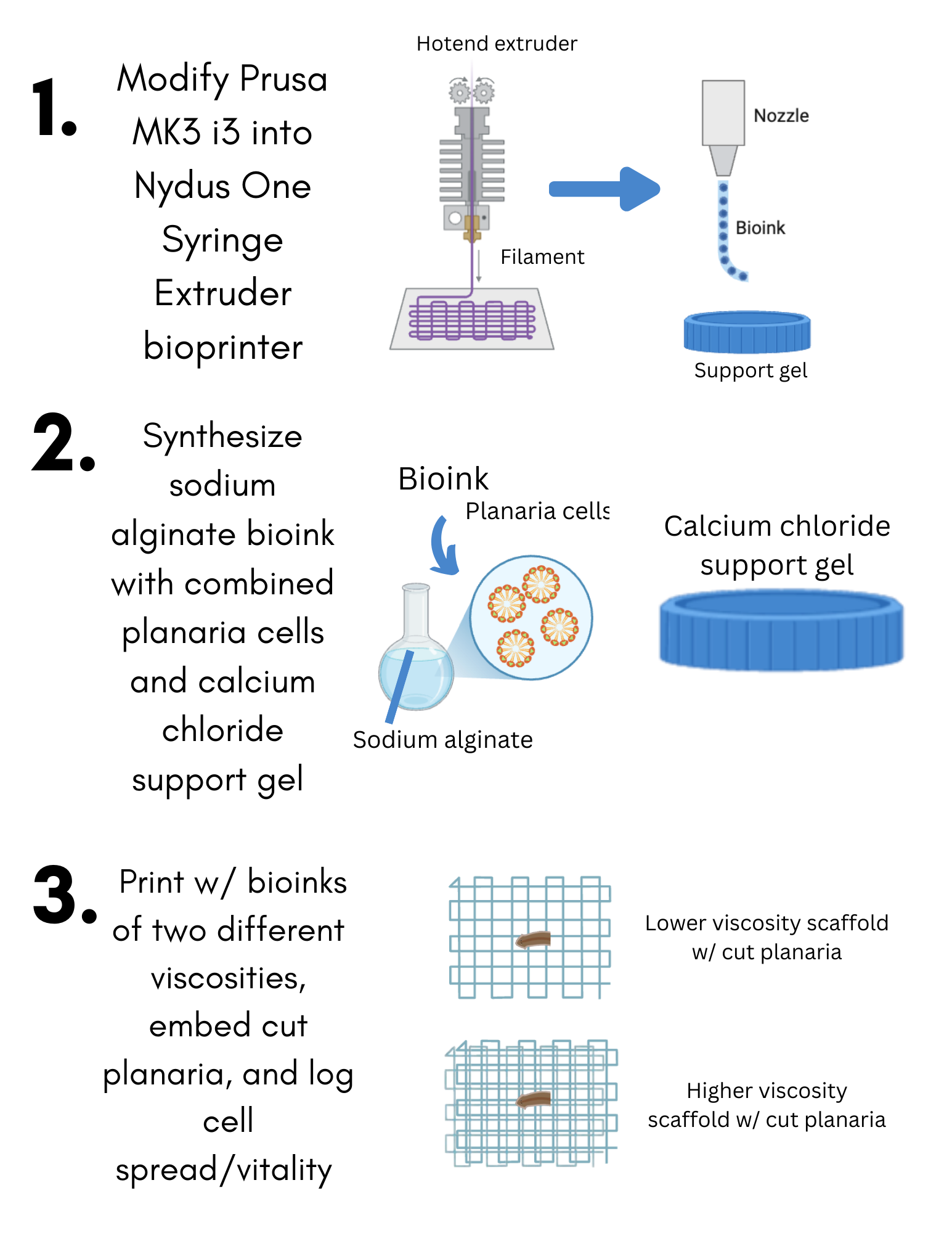
Using sodium alginate, calcium chloride, and isolated planarian cells, two trials with varying print parameters can
be designed to mimic a natural cancer microenvironment. By comparing cellular regeneration in these trials to that of
a control group, the most effective bioprint conditions can be identified. This successful model provided accuracy in
rebuilding the environment. Overall, both trials showed promise for future applications of metastasis research, and future work will
focus on resimulating the environment without material parameters present in this study.

 -->
-->
 -->
-->