STEM I
Filtering Microplastics Using a Semipermeable Membrane Filter
Abstract
During the past 75 years, plastic production has increased from
1.5 million tons to 322 million tons a year because of the cost
efficiency and variety of uses. Due to the increased production, many
plastics are improperly disposed of and enter the environment. Every
year, 8 million tons of plastic enters the ocean and eventually
breaks down into microplastics which are less than 5 mm in size. At
this point, these plastics can be consumed by ocean organisms and
harm them when entering animal tissue. To combat the influx of
microplastics into the ocean, a filter with a semipermeable membrane
was used to extract microplastics from samples of water, as a
possible method to eliminate plastic waste from entering the water
supply. Only materials that are smaller than the pores in the
membrane could pass through, so the membrane can be used to eliminate
as much plastic as possible from a sample of water. A
spectrophotometer was used to measure the amount of light that was
absorbed by the plastics. Then the concentration of the plastics can
be compared. The change in the absorbed light determines the amount
of plastic that is removed when filtered through the membrane. This
paper presents that the concentration of microplastic decreased by at
least 30% when put through the different prototypes made from 2
different filters. These filters can eventually be applied to
household items like the washer to prevent microplastics from
entering the sewage system and eventually entering the ocean.
Phrase 2
The aim of this project is to engineer a reusable apparatus
that is portable and filters microplastics from water efficiently.
Phrase 1
Millions of particles of microplastics pollute the environment
from human activity. Although they can be filtered out, it is
difficult to do so efficiently.
Background

There are millions of particles of microplastic in the ocean
and other bodies of water. Plastic comes from many of common plastic
items in commercial products. With the variety and usefulness of
plastics, it is not a surprise that many products in the market are
made from plastic. However, with the popularity of this material,
came the abundance of litter that now invades the environment.
Plastics serve various purposes and are cheap to manufacture, so
within the 100 years that it has been created, it has become a staple
material in many commercial products. An estimated 4 to 12 million
tons of plastics is estimated to escape into marine environments from
2010 alone which plastics to collect in the ocean and other bodies of
water (Coppock et al, 2017). When plastic wears down, it breaks down
physically into smaller and smaller pieces, and are transported into
waterways. Plastics are polymers which are composed of smaller units,
so its molecular structure stays the same while it physically gets
smaller.
There is so much microplastic litter that there is a big patch
of microplastic in the Pacific Ocean, known as the Great Pacific
Garbage Patch (The Great Pacific Garbage Patch, n.d.). Due to ocean
currents, plastic particles are gathered in the Pacific Ocean to
create a mass of plastic particles that weighs tons. The Great
Pacific Garbage Patch isn’t the only collection of plastic, others
exist too where ocean currents gather them. There has also been
plastic found further down in the water than just on the surface.
Deep sea sediments have also been found to contain microplastics.
This poses an issue since it is much harder to filter plastic found
that far down due to the ocean life dwelling there.
Microplastic pollution is contaminating the environment and
many of the foods people consume. Not only can microplastic get into
human and animal tissue, it also acts as a medium to absorb other
pollutants which may cause even more damage to wildlife and humans
(Van Cauwenberghe, Vanreusel, Mees, & Janssen, 2013). Microplastics
concentrate pollutants, thus increasing their toxicity. For example,
an increase of salinity increases the adsorption capacity of
microplastics (Ma, Zhao, Zhu, Li, & Yu, 2019). This means that when
microplastics reach the ocean, they can exacerbate the toxicity of
other present pollutants. When microplastic can get into the human
body, jagged edges can cut tissues and cause inflammation (Sun, Dai,
Wang, van Loosdrecht, & Ni, 2018). There may be no immediate threats
to having microplastics in our tissues, and most plastics typically
pass through the digestive system, but the build up of plastics in
the body can release toxins.
Wastewater treatment plants are one of the culprits that
release microplastics into the environment. When water is treated,
most of the microplastics are filtered out and end up in sludge. This
sludge can be used as fertilizer and other products which releases
the microplastics into the environment where they end up in our
marine biomes. Of the microplastics found in the wastewater treatment
plants, microplastic fibers are much more common than microplastic
particles (Lares, Nicbi, & Sillanpaa, 2018). This poses a problem
because they are much harder to filter due to the fact they can pass
through many mesh filters. Meshes bigger than 250 nanometers allows
too many small particles to pass through (Oßmann, Sarau, Schmitt, et
al. Anal Bioanal Chem 2017).
The issue with filtering microplastics is that there are many
types of plastics and those different plastics which have different
shapes and sizes. Most define microplastic as plastic particles that
are less than 5 millimeters. Plastics have different densities which
makes filtering by density difficult. However, a semipermeable
membrane can be used to filter out as many microplastics as possible.
With different pore sizes, only microplastics of larger size will be
able to pass through the filter. By passing a sample of water and
microplastics through the filter, microplastics can be filtered out
and the remaining concentration of microplastics can be analyzed.
Procedure

A sample of microplastic will be made before filtration can
occur. Deionized will be used to ensure there are no other
preexisting particles in the end sample of water. The water will be
placed in a beaker and heated over a hot plate. A cut tea bag will
then be placed into the water with a magnetic stirrer. This will
break down the plastic into some microscopic pieces. After 10 minutes
any large plastic pieces are removed and the sample can be filtered.
The concentration of the microplastics can be measured with a
spectrophotometer. Different filtration apparatuses will be used
cutting different types of membrane filters so they will fit into a
reusable filter holder. A syringe will then hold an amount of the
sample plastic water to be passed through the filter. The
concentration of the plastic will be measured with a
spectrophotometer and then compared to the starting concentration.
Create a sample of microplastic:
Heat water until 80 degrees celsius.
Cut a tea bag into strips and place it into the water.
Add a magnetic stirrer to stir the water.
Wait 10 or 20 minutes then remove the large bits of teabag
Measure the amount of microplastic present in that sample using
spectrophotometer.
Making and using the filter:
Make the filtration device with membrane filters that have pore
sizes less than 1 micrometer. Cut them so that the membranes fit
inside the filter holder.
Filter the sample of water through the device by using a
syringe to push the water past the filter.
Measure the concentration of microplastic that is filtered out
with the spectrophotometer.
Results
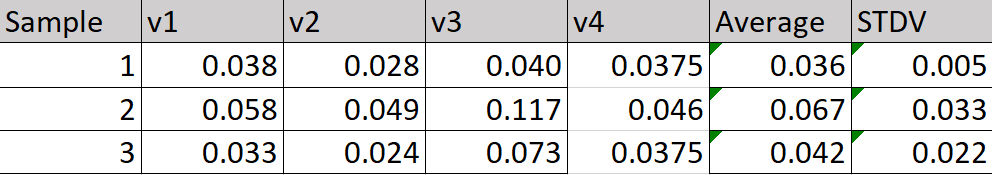
After testing the prototypes, the sample optical density was
obtained. At a wavelength of 375 nm all the absorbance was lower than
0.100. The prefiltered microplastic samples were tested each time the
prototypes were tested. The average absorbance for the first sample
was 0.036. The average for the second sample was the highest at
0.067, and the last sample had an absorbance of 0.042 as seen in
table 1.
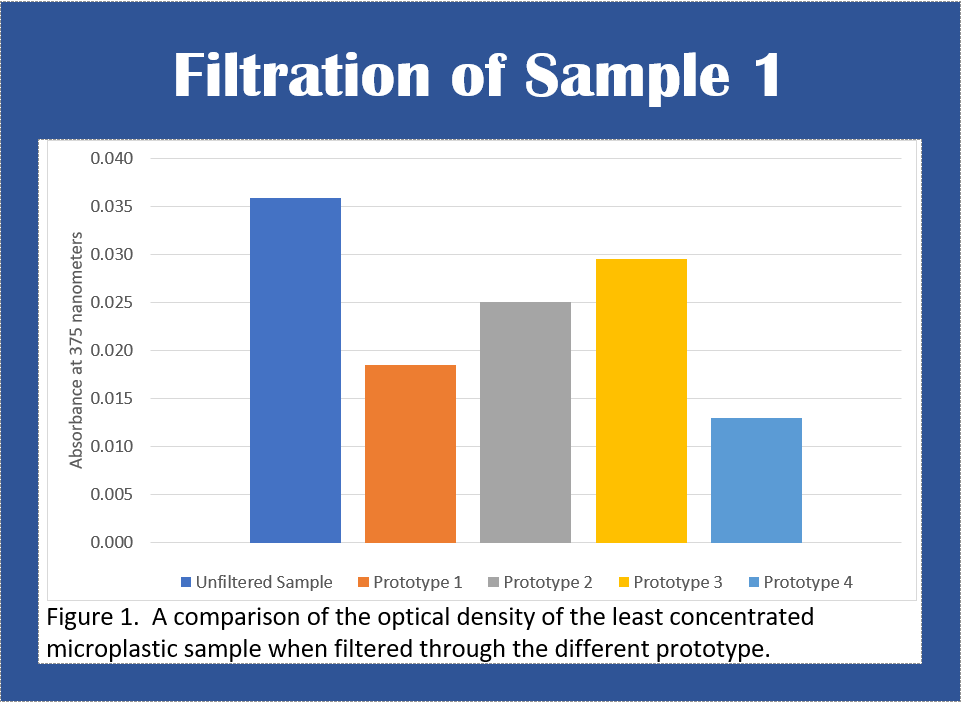
Figure 1 shows that after the sample was filtered, prototype 1
was able to filter out 49% of the microplastic contents, prototype 2
was able to filter out 31% of the contents, prototype 3 was able to
filter out 18% of the contents and prototype 4 was able to filter out
64% of the microplastics.
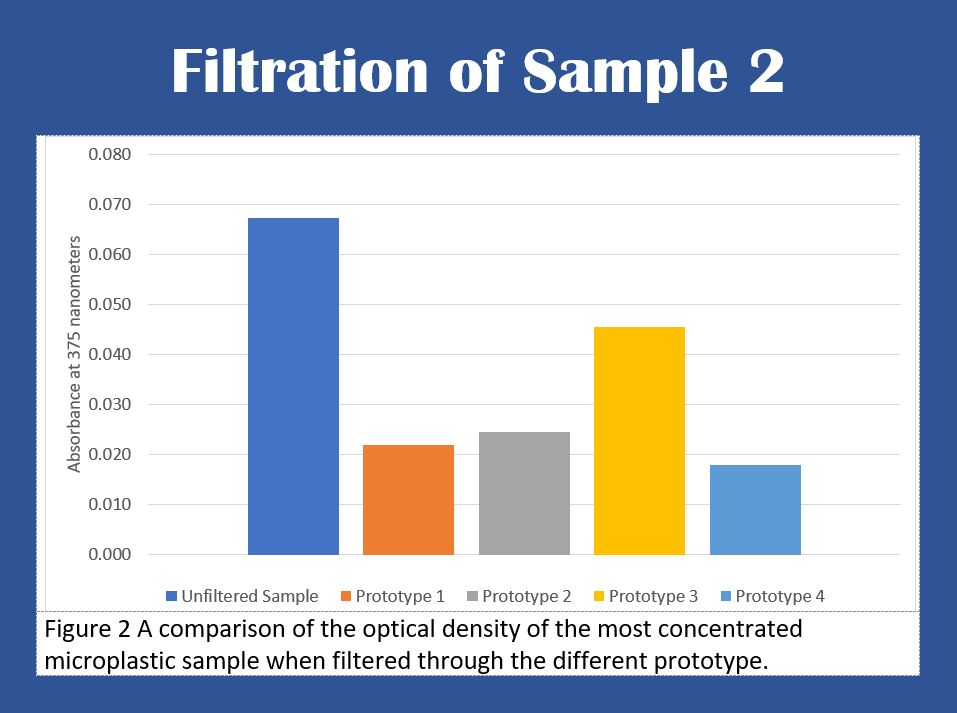
Figure 2 shows that after the sample was filtered, prototype 1
was able to filter out 67% of the microplastic contents, prototype 2
was able to filter out 63% of the contents, prototype 3 was able to
filter out 34% of the contents, and prototype 4 was able to filter
out 73% of the contents.
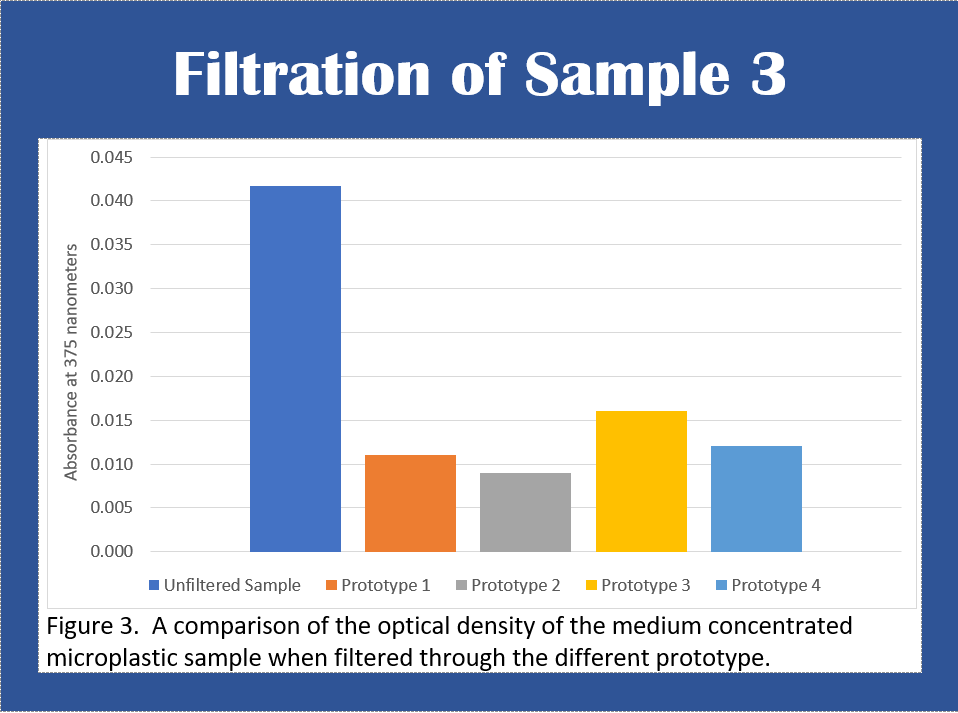
Figure 3 shows that after the microplastic sample was filtered,
the first prototype filtered 74% of the microplastics, the second
prototype was able to filter 79% of the contents, the third prototype
was able to filter out 62% of the contents and the fourth filtered
71% of the microplastics.
After the optical densities were found, pair t-tests were used
to see if there were any significant differences between the
absorbance of the different prototypes. The tests revealed that
between prototypes 1, 2, and 4 the p value was consistently over
0.05, and between prototype 3 and 4 the p value was 0.02. This
indicates that there exists a significant difference between
prototype 3 and rest of the prototypes.
Discussion/Conclusion
When making the microplastic samples, time was the greatest
factor in determining the concentration of the created microplastic
water. From table 1, the concentration of the first and third
microplastic samples were similar because they were both left on the
stir plate for the same amount of time (10 min). The third sample
with an average absorbance of 0.042 may have been higher due to the
smaller pieces the tea bags were cut into. The second sample was left
on the stir plate for 20 minutes which resulted in the highest
microplastic concentration.
From the testing, it is shown that prototype 4 filtered out
more microplastics than in prototypes 1, 2 and 3. When filtering the
low concentrated microplastic sample, prototype 4 with all 3 of the
membrane filters is able to filter out 36% more of the microplastic
solution than the third prototype, 33% more than the second
prototype, and 15% more than the first prototype.
When testing the prototypes on the high concentrated
microplastic sample, the fourth prototype again filtered out 39% more
of the microplastics in the sample than the third and only 10% more
than the second prototype and 6% more than the firsts. All tests
showed that, overall, the third prototype by far performed the worst,
while prototypes 1, 2, and 4 had similar results with prototype 4
being a bit more effective than the second and the third. The t-tests
also support this idea because only prototype 3 was seen with any
statistical difference, while the other 3 prototypes didn’t have a
statistical difference.
Prototype 4 was the most effective at filtering because it
contained all three types of membrane filters that were used among
the prototypes. However, in terms of resources, prototype 1 is the
most efficient. With the larger pore sized membranes, it is the
cheapest filter to make.
Prototype 3 was made of membranes with a pore size of 0.45
micrometers and 0.2 micrometers which are smaller than the membranes
in prototype 1 and 2. This means that a device with these smaller
pore sizes may not necessarily cause a great change in the number of
particles filtered out. This means that costs for buying the membrane
may be saved when not getting the smallest pore size membrane filter.
References
Ashter, S. A. (2014). 3 - Review of Characteristics of Common
Plastics for Thermoforming. In Thermoforming of Single and Multilayer
Laminates (pp. 39–63). doi:
https://doi.org/10.1016/B978-1-4557-3172-5.00003-7
Coppock, R. L., Cole, M., Lindeque, P. K., Queirós, A. M., &
Galloway, T. S. (2017). A small-scale, portable method for extracting
microplastics from marine sediments. Environmental Pollution, 230,
829-837. doi:10.1016/j.envpol.2017.07.017
Covernton, G. A., Pearce, C. M., Gurney-Smith, H. J., Chastain,
S. G., Ross, P. S., Dower, J. F., & Dudas, S. E. (2019). Size and
shape matter: A preliminary analysis of microplastic sampling
technique in seawater studies with implications for ecological risk
assessment. Science of the Total Environment, 667, 124-132.
doi:10.1016/j.scitotenv.2019.02.346
Kirstein, I. V., Kirmizi, S., Wichels, A., Garin-Fernandez, A.,
Erler, R., Loder, M., & Gerdts, G. (2016). Dangerous hitchhikers?
Evidence for potentially pathogenic Vibrio spp. on microplastic
particles. Marine Environmental Research, 120, 1–8. doi:
https://doi.org/10.1016/j.marenvres.2016.07.004
Lares, M., Ncibi, M. C., & Sillanpaa, M. (2018). Occurrence,
identification and removal of microplastic particles and fibers in
conventional activated sludge process and advanced MBR technology.
Water Research, 133, 236–246. doi:
https://doi.org/10.1016/j.watres.2018.01.049
Lenz, R., & Labrenz, M. (2018). Small Microplastic Sampling in
Water: Development of an Encapsulated Filtration Device. Water,
10(8). doi: https://doi.org/10.3390/w10081055
Lv, L., Qu, J., Yu, Z., Chen, D., Hong, P., Sun, S., & Li, C.
(n.d.). A simple method for detecting and quantifying microplastics
utilizing fluorescent dyes - Safranine T, fluorescein isophosphate,
Nile red based on thermal expansion and contraction property.
Environmental Pollution, 225. doi:
https://doi.org/10.1016/j.envpol.2019.113283
Ma, J., Zhao, J., Zhu, Z., Li, L., & Yu, F. (2019). Effect of
microplastic size on the adsorption behavior and mechanism of
triclosan on polyvinyl chloride. Environmental Pollution, 254(Pt B),
113104. doi:10.1016/j.envpol.2019.113104
Mai, L., Bao, L., Shi, L., Wong, C., & Zeng, E. (2018). A
review of methods for measuring microplastics in aquatic
environments. Environmental Science and Pollution Research, 25(12),
11319-11332. doi:10.1007/s11356-018-1692-0
Oßmann, B.E., Sarau, G., Schmitt, S.W. et al. Anal Bioanal Chem
(2017) 409: 4099.
https://doi-org.ezproxy.wpi.edu/10.1007/s00216-017-0358-y
Sun, J., Dai, X., Wang, Q., van Loosdrecht, M. C. M., & Ni, B.
(2019). Microplastics in wastewater treatment plants: Detection,
occurrence and removal. Water Research, 152, 21-37.
doi:10.1016/j.watres.2018.12.050
Van Cauwenberghe, L., Vanreusel, A., Mees, J., & Janssen, C. R.
(2013). Microplastic pollution in deep-sea sediments. Environmental
Pollution, 182, 495-499. doi:10.1016/j.envpol.2013.08.013
Wilkinson, T. L., & Sork, F. J. (n.d.).
Febuary Fair Poster
Here is the information I had for the fair.





