2025 MSEF Project
NeuroLens: Multimodal Deep Learning Application for Effective Multi-Class Classification and Semantic Segmentation of Invasive Brain Tumors
Abstract
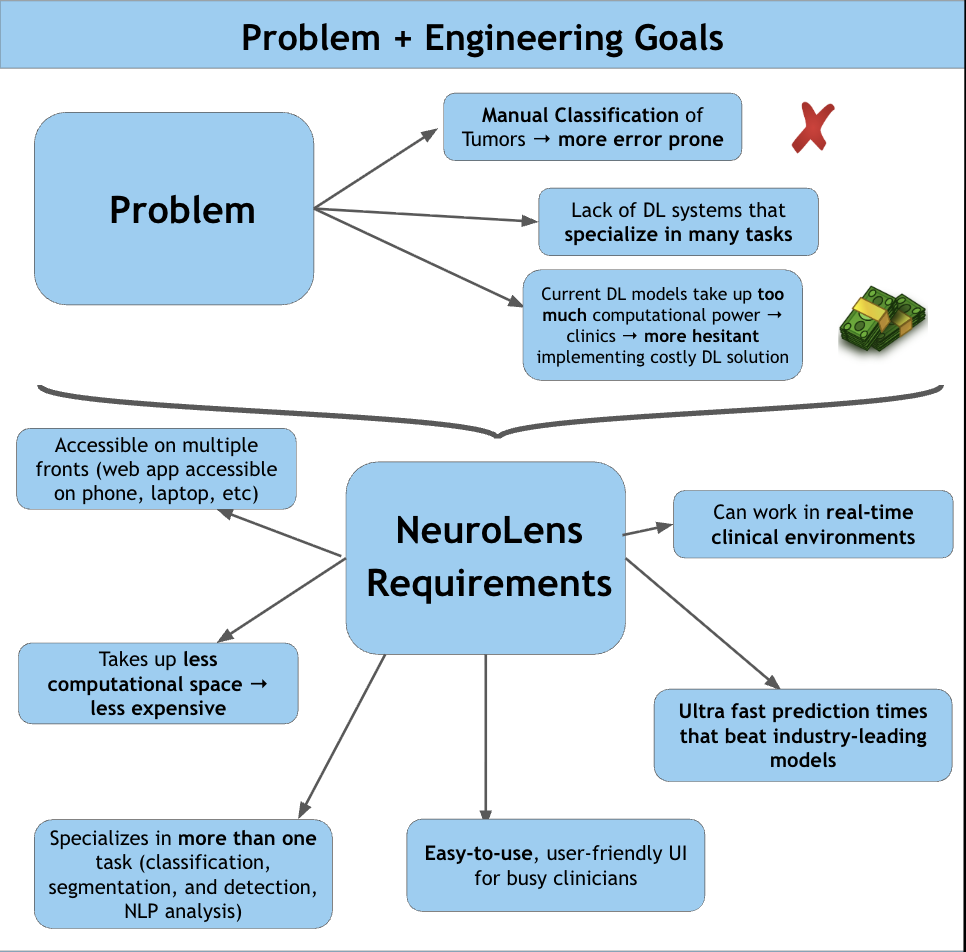
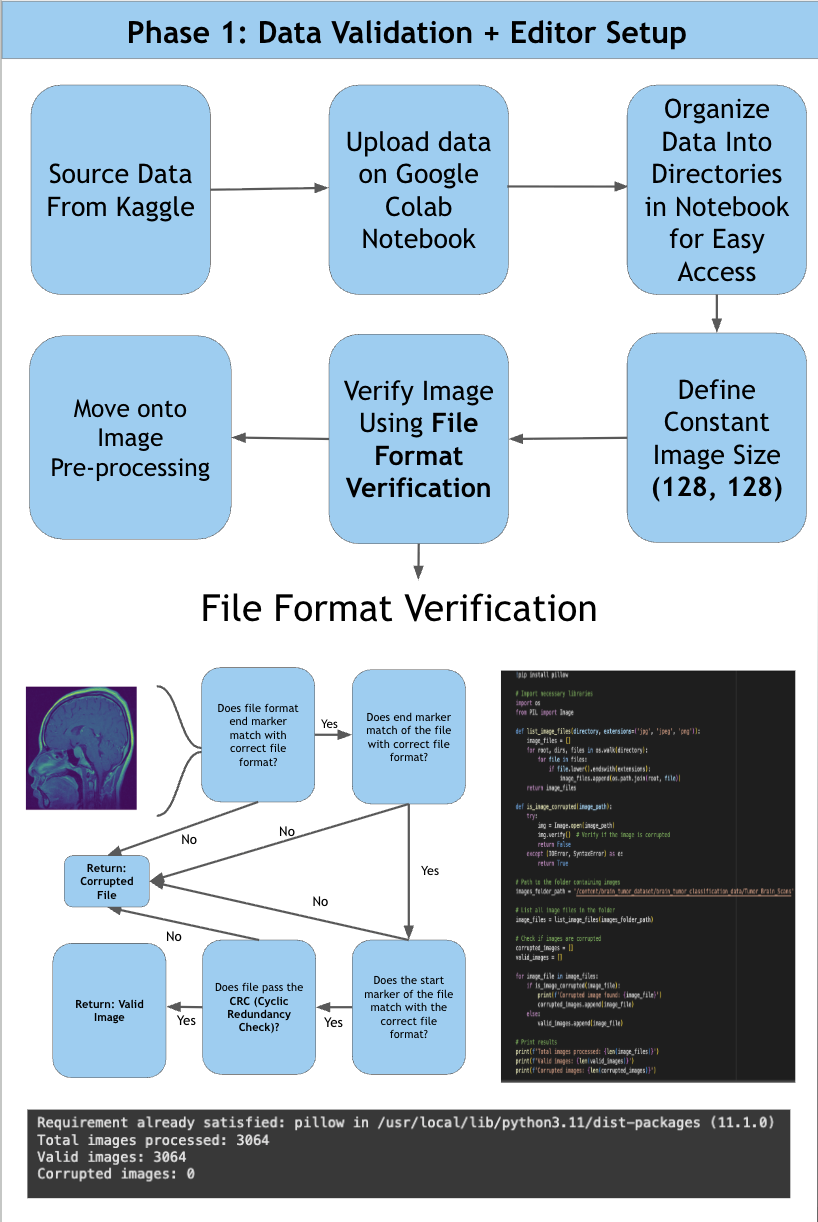
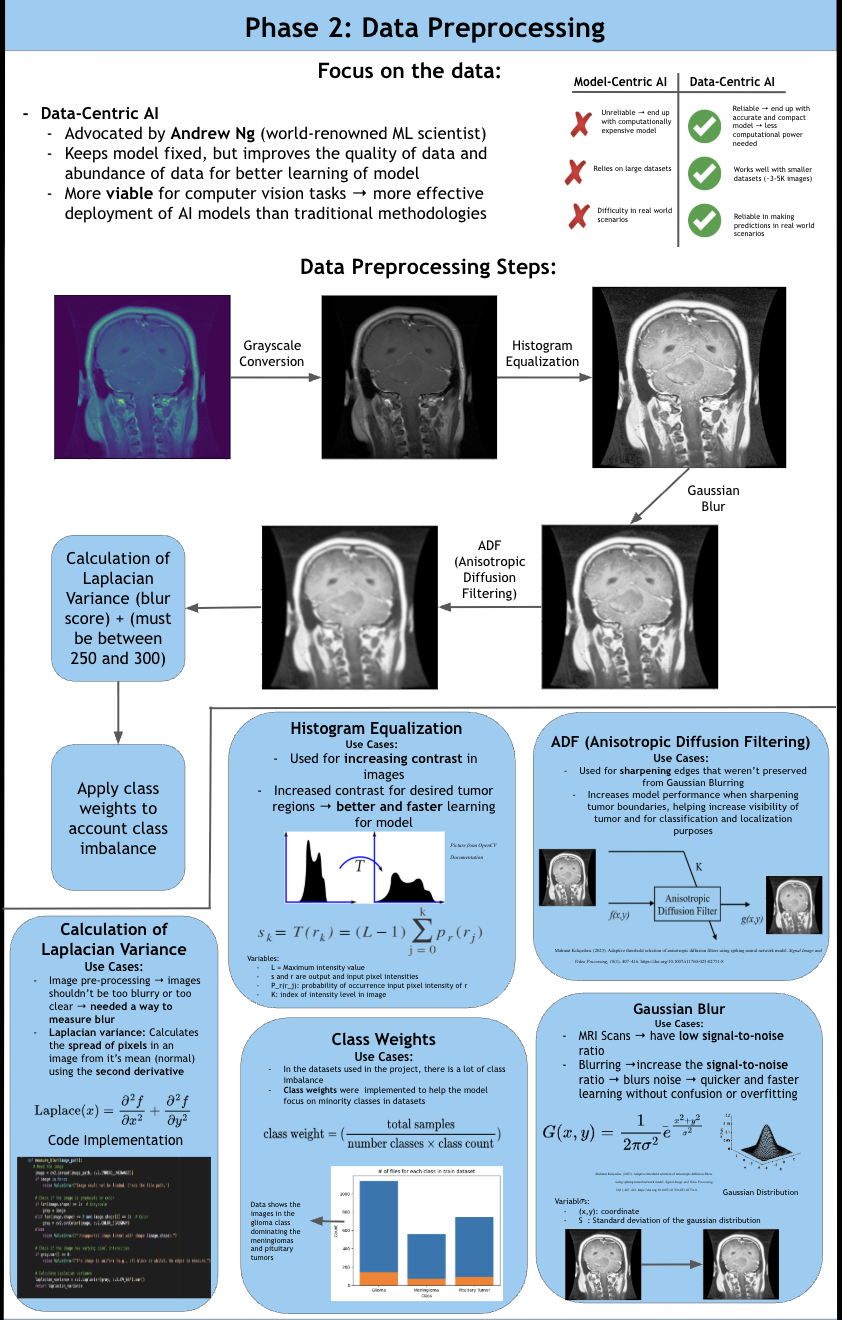
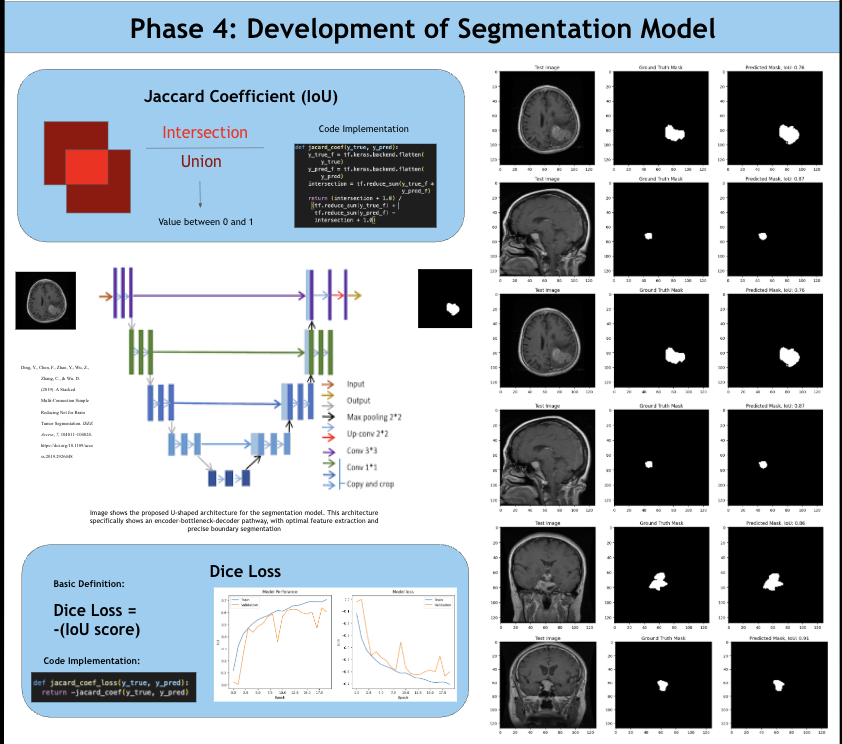
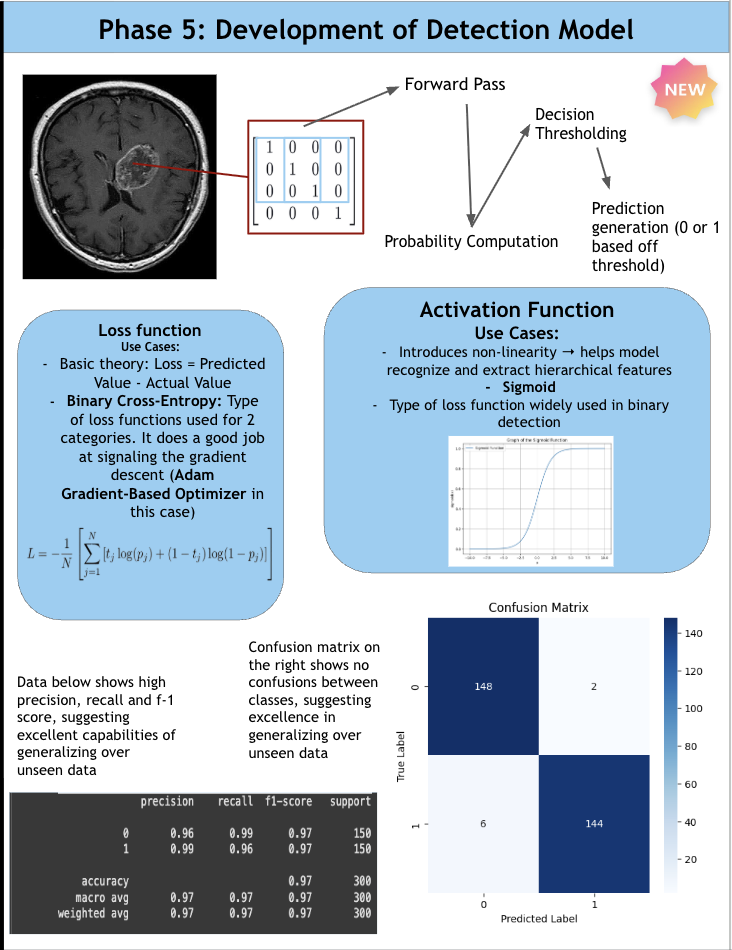
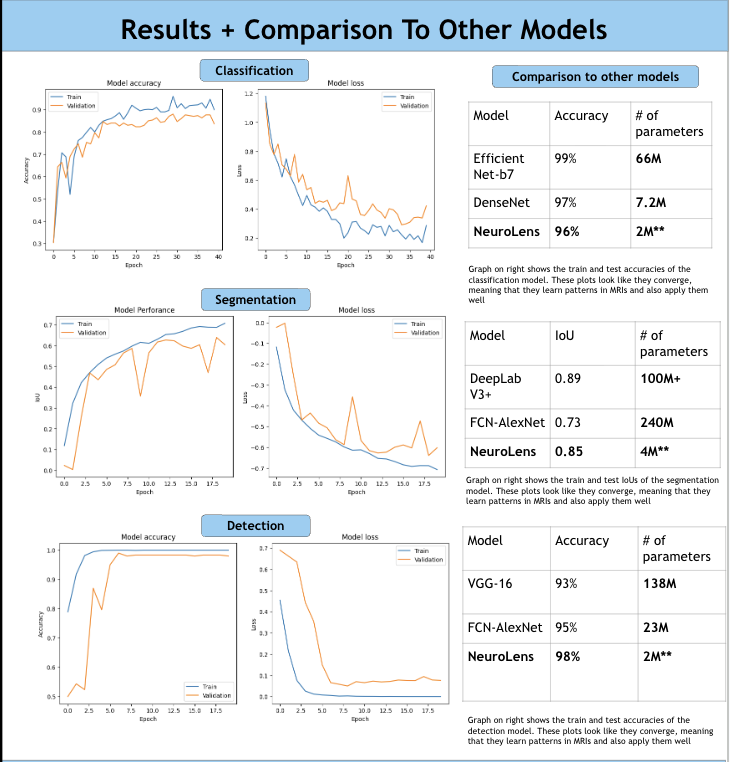
According to the American Brain Tumor Association, around 90,000 people get diagnosed with brain tumors every year. Brain tumors are masses of abnormal cell growth in the brain caused by DNA mutations or other genetic factors. There are two types of tumors: non-cancerous and malignant. This study focuses on categorizing and locating malignant brain tumors like gliomas, pituitary tumors, and meningiomas. In the clinical world, quick and accurate diagnoses are essential, and manual classification can be prone to errors and very time-consuming. Current deep-learning models tend to use large amounts of computational power, yet yield average results. For this reason, NeuroLens was created. NeuroLens provides multimodal features that no other app has. It can accurately detect, classify and segment brain tumors from MRI scans within seconds, compared to larger models that take more time. An AI chatbot called NeuroLens Assistant is also implemented for further diagnosis and prognosis of results given by NeuroLens. NeuroLens first uses special image preprocessing techniques like ADF (Anisotropic Diffusion Filtering) to increase the sharpness of images and class weights that guide the model’s attention to minority classes. Additionally, it uses techniques like Gaussian Blur and Histogram Equalization in parallel. A custom binary mask is then employed for further refining of important features, increasing the analytical preciseness and timely diagnosis of invasive tumors. The blurriness of the image was then measured using a constant called the laplacian variance. Images with an ideal blur score of around 250-300 are essential when fed to the classification model. Then, scaffolds of models were created and trained on their respective datasets. These scaffolds were then built upon either by adding more layers or adding regularization techniques like batch normalization and dropout layers to prevent overfitting and preserve the learning of the model until they presented high performance metrics. Additionally, another problem that is faced is the ambiguity of MRI scans. It may be unclear to the surgeon where the tumor is for excision based solely off the scan. That is where a highly sophisticated segmentation model can be most useful. This app uses an MRI scan and mask dataset from multiple sources on the Kaggle database. The segmentation model in this app was a custom, robust UNet with an encoder, bottleneck, and decoder pathway. This segmentation model was then trained, validated and iterated upon extensively. NeuroLens presented with extraordinary performance metrics. The train accuracy for the binary detection model was 99%, while the test accuracy was 96%. The train, test, and validation accuracies for the classification model was 99.2%, 96.42% and 95.77% respectively. The average IoU (intersection over union) for NeuroLens’ segmentation model, a statistical measurement on a scale of 0-1 of how much of the model’s predicted mask overlaps with the true mask, ranged from 0.71-0.91, close to industry-leading segmentation models with only a fraction of their parameters. These results underscore the potential of NeuroLens as a ground-breaking tool to deliver high-precision detection, classification, and segmentation of brain tumors in a clinically relevant environment, demonstrating its vitality in real-world medical applications.
Project Outline