Graphical Abstract
A class that simulates an actual research process with the STEM project. Thus, the class is an effective preparation for introducing the student into the scientific world of research. The STEM Project is an independent research project where the student creates their own research questions or engineering objectives, and they would do experiments to answer said questions, or build a product for an engineering project. All the work is in preparation for the February Fair, where the students will have to present their projects.
STEM Title: Utilizing Saccharomyces cerevisiae to develop a system of mutualistic wastewater treatment with both chemical and biological treatments
Overview: Modern wastewater treatment is not affordable as it requires a lot of money to buy the materials, maintain them, and dispose of the sludge left after the reaction. Therefore, my project uses yeast, a very economical organism that is widely available on the market and one that does not produce toxic sludge that may poison the water after treatment. Treatment with yeast will be combined with treatment with chitosan harvested from the yeast to create a chemical treatment pretreatment that does not produce toxic waste either.

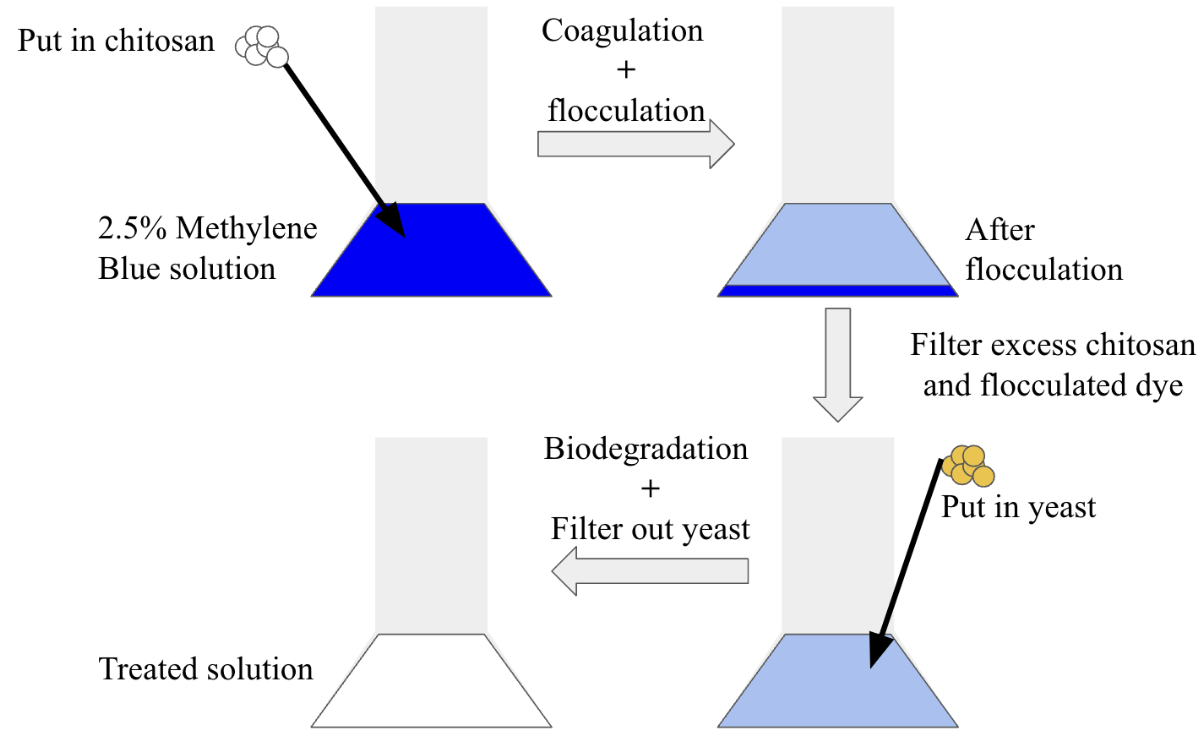
The textile industry’s untreated dyes being released into the environment is one of the major causes of freshwater pollution. These dyes are toxic to animals, and they can block out sunlight from reaching underwater vegetation, which is not able to effectively photosynthesize, so the ecosystem is damaged. Hence, wastewater treatment is necessary to preserve the resource and to protect the environment. However, current treatment methods are flawed due to the copious amount of energy spent, the need for tremendous volumes of materials used during the process, and harmful byproducts released after treatment. Therefore, the overall aim of this project is to develop a cheaper treatment method for removing waste from textile effluents by using Saccharomyces Cerevisiae as the primary material for this system. The fungus was chosen due to its availability, cheap pricing, and eco-friendliness. There are two treatment methods within this system: a chemical treatment and a biological treatment. The first treatment method used chitosan harvested from yeast for coagulating and flocculating the dye, which was removed by using a filter. Then, the biological treatment commenced as the yeast biodegraded the remaining methylene blue dye within the solution. The effectiveness of this method was tested by purifying 1L of a 2.5% concentrated methylene blue dye solution. This method removed more than 99% of methylene blue dye from the solution over many trials, showing the effectiveness of the system. The results of this project could help in developing a cost-effective and yeast-based decolorization method at wastewater treatment facilities.
The goal of this project was to create a cheap wastewater treatment system for treating methylene blue dye, and further assess yeast’s adaptability and effectiveness in treating methylene blue dye.
What is the effectiveness of yeast in biodegrading methylene blue dye under non-optimized conditions and what is the effectiveness of chitosan obtained from yeasts at coagulating methylene blue dye?
Effluents produced by the textile industry can be very detrimental to the environment and human health (Al-Tohamy et al., 2022)
Toxic dyes contained within the discharge could remain within the earth and water for an extended period of time.
On land, the dyes damage the environment by degrading the soil’s quality and absorbing large amounts of oxygen, causing plants to wither due to lack of nutrition.
In the water, dyes can inhibit sunlight from passing through to algae, decreasing the amount of oxygen produced at this location, and this effect can cause algae to expire, and animals to starve or be poisoned by the toxin.
Toxic dyes contaminate the drinking source for humans, which when consumed, causes several diseases such as irritation, dermatitis, and even cancer.
Hence, treatment of textile wastewater is needed to preserve the environment, ensure safety for humans, and maintain a clean water source for consumption and utility.
Current textile wastewater treatment methods are expensive and consume a large amount of energy, which makes the technology inaccessible to low-income governments (Al-Tohamy et al., 2022).
Organic alternatives to current treatments are not only cheaper, but they are also much more environmentally friendly as previous treatment methods may also leave toxic chemicals in the wastewater after treatment (Zahrim et al., 2011).
The organic polymer that will be used in this research is chitosan as it had been proven to remove 98% of methylene blue dye in a simulated wastewater solution under optimal conditions (Karthi et al., 2022).
The microorganism that were used for biodegradation in the experiment is Saccharomyces cerevisiae due to its resilience to dye, ability to produce chitin, and ability to effectively degrade inorganic dyes in effluents (Ghodsi et al., 2024).
Hence, a economically effcient system for wastewater treatment is possible using chitosan and yeast
*Refer to graphical abstract for treatment method
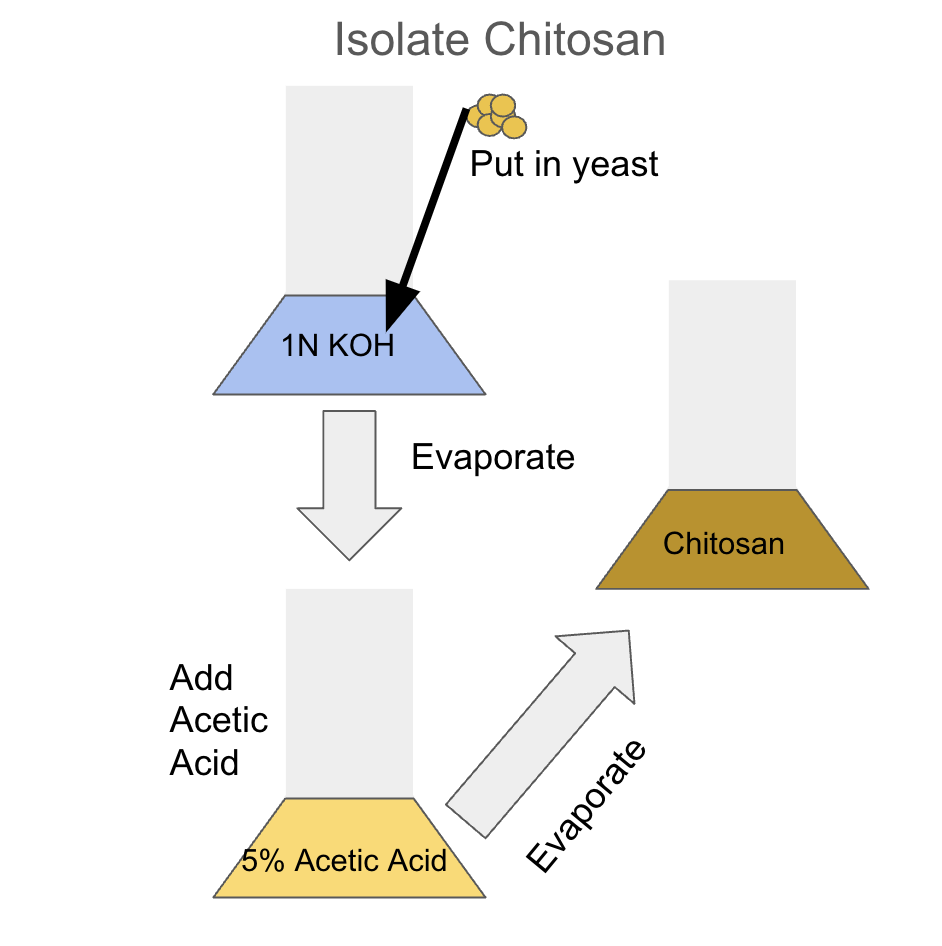
Determine the effectiveness of the chitosan at coagulation and flocculation extracted from the cell wall of Saccharomyces cerevisiae. The objective is to produce chitosan from the yeast to be as effective as pure chitosan bought from a supplier and chitosan extracted from crustaceans. Our approach is to dehydrate the yeasts and put them in a solution of hot sodium hydroxide and stir it. Then, separate the yeast from the solution and wash them with distilled water (Syala et al., 2024). Next, dry the cells and put them in a hot sulfuric acid solution. Use a filter paper to extract the liquid for cooling. Then, remove the chitosan from the solution with centrifugation. Next, compare the chitosan obtained from the fungus to pre-made chitosan under the same conditions. Spectroscopy analysis will determine the effectiveness of the isolated chitosan by assessing the degree of deacetylation.
Determine the effectiveness of biodegradation of methylene blue dye by Saccharomyces cerevisiae. The objective is to highlight the potential of yeast to be used as a biological treatment for textile effluents. Our approach is similar to the previous procedure set for comparison between different sources of chitosan for coagulation. We would prepare 2.5% concentrated methylene solutions, and half of them will be treated with yeast. All solutions in this experiment will be under the same conditions as the chitosan treatment. After the reaction had completed, centrifuge the samples to obtain the supernatant in preparation for spectroscopy. Compare the absorbance of the treated mixture and the control, untreated solution to determine the removal rate of methylene blue dye due to the yeast.
Determine the effectiveness of the combined system for treating a simulated wastewater solution, which is a 5% concentrated methylene blue dye (Yaseen and Scholz, 2018). Our approach is the same as the procedure mentioned for specific aim #2, with the difference that the chemical treatment will be performed before the biological treatment. Chitosan will be added to 10 of the solutions created, and the same procedure will take place, but after the reaction is done, the flocculated dye will be filtered out, and 1.5g of yeasts will be added into the solution. After the reaction is complete, spectroscopy analysis will be performed to assess the effectiveness of the system as a whole.
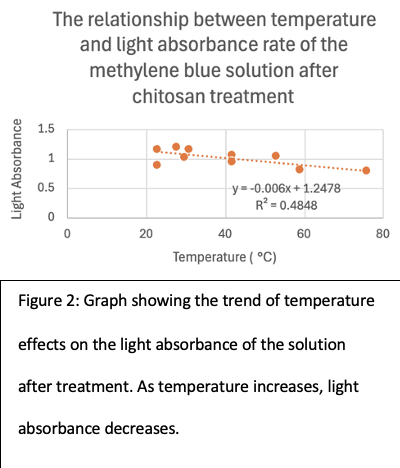
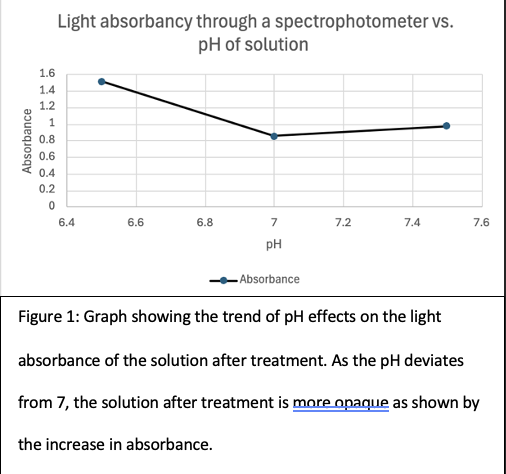
Figure 1 shows that the differences between making the solution more acidic and more basic are distinct. This is surprising as a different slope is expected for a more acidic and a more basic solution is expected, but the slope is opposite to what is expected, as a more acidic solution should perform better than a basic solution from past research. This is because chitosan is slightly basic, so a slightly acidic solution would still have a decent electrostatic force for the molecules to be coagulated with chitosan as opposed to the basic solution that will repel the methylene blue dye from the coagulant. This experiment allows further testing on coagulation by chitosan to be done at 7 pH to achieve the optimal level.
Figure 2 implicitly shows the relationship between the effectiveness of coagulation by chitosan and the temperature of the solution. This result is to be expected as a higher temperature promotes more interaction between the dye particles and the chitosan, which allows the chitosan to coagulate more dye in a lesser amount of time. The gradual downward slope is also expected as temperature is frequently discussed as the least important factor in promoting chitosan coagulation. The correlation coefficient of 0.70 shows a moderate relationship between the temperature and the absorbance of the MB dye solution after treatment. This low correlation value is attributed to errors in running the experiment, most notably, the use of a very concentrated dye solution of 2.5% with only 30 minutes of contact time for the chitosan to coagulate. This makes it so that the temperature difference does not show obvious deviations from each other because the time for the reaction is too short for the chitosan to coagulate meaningfully.
External variables are controlled meticulously, including methylene blue dye concentration, contact time, and the amount of chitosan in each solution. The methylene blue dye concentration in each solution is obtained by pipetting 1 mL of 2.5% concentrated methylene blue dye solution and measuring the 1 mL with a graduated cylinder. The distribution of methylene blue dye in the 2.5% solution is homogenous, so the 1 mL obtained from it will be consistent with all samples. The contact time for each solution is measured with a stopwatch, and the experimentation stops after 30 minutes with some negligible differences in seconds. Then, each sample are allowed to settle for the same time, under the same conditions, and spectroscopy analysis was done with only the supernatant sample that is then centrifuged to not provide more time for the chitosan to coagulate, as the chitosan was separated from the solution. The amount of chitosan in each solution was weighed to be exactly 1.00g, and the chemical was put into the solution carefully as to not spill the chitosan.
These experimentations are to determine the validity of past studies, and both had been supportive of past research. The most effective pH for chitosan to coagulate is 7, and the chemical will perform better at higher temperatures (Karthi et al., 2022). Thus, coagulation by chitosan could be done in optimal conditions that was determined after these experiments.
Cost analysis had not been done at this time, nor had the effectiveness of yeast be measured.
The objective of the research is to determine the effectiveness of S.cerevisiae in contributing to a wastewater treatment system. This is done by using the yeast itself to biodegrade and treat methylene blue dye in a concentrated solution, extracting chitosan from the yeast, and subsequently determining the chemical’s ability to flocculate the dye from the solution. Results in the success of the treatment system had not been obtained at this time, but the optimal conditions for chitosan coagulation is determined, so each future test will show the chitosan’s full capabilities in coagulating MB dye. The success of the system shows that wastewater treatment can be accessible to all governments, so clean water in the environment can be easily maintained. Thus, the human impact on the environment will be lessened, pollution will be reduced significantly, and access to clean water for human activities will be more readily available.
Adane, T., Adugna, A. T., and Alemayehu, E. (2021). Textile industry effluent treatment techniques. Journal of Chemistry, 2021, 1–14. https://doi.org/10.1155/2021/5314404
Al-Tohamy, R., Ali, S. S., Li, F., Okasha, K. M., Mahmoud, Y. A.-G., Elsamahy, T., Jiao, H., Fu, Y., and Sun, J. (2022). A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicology and Environmental Safety, 231, 113160. https://doi.org/10.1016/j.ecoenv.2021.113160
Araújo, D., Ferreira, I. C., Torres, C. A., Neves, L., and Freitas, F. (2020). Chitinous polymers: Extraction from fungal sources, characterization and processing towards value-added applications. Journal of Chemical Technology and Biotechnology, 95(5), 1277–1289. https://doi.org/10.1002/jctb.6325
Earth’s freshwater. Education. (n.d.). https://education.nationalgeographic.org/resource/earths-fresh-water/
Environmental Protection Agency. (2024, November 18). Sources and Solutions: Wastewater. EPA. https://www.epa.gov/nutrientpollution/sources-and-solutions-wastewater
Gachhi, D. B., and Hungund, B. S. (2018). Two-phase extraction, characterization, and biological evaluation of chitin and chitosan from Rhizopus oryzae. Journal of Applied Pharmaceutical Science, 8(11), 116–122. https://doi.org/10.7324/japs.2018.81117
Ghodsi, S., Kamranifar, M., Fatehizadeh, A., Taheri, E., Bina, B., Hublikar, L. V., Ganachari, S. V., Nadagouda, M., and Aminabhavi, T. M. (2024). New insights on the decolorization of waste flows by Saccharomyces cerevisiae strain – A systematic review. Environmental Research, 249, 118398. https://doi.org/10.1016/j.envres.2024.118398
Hosney, A., Ullah, S., and Barčauskaitė, K. (2022). A review of the chemical extraction of chitosan from shrimp wastes and prediction of factors affecting chitosan yield by using an artificial neural network. Marine Drugs, 20(11), 675. https://doi.org/10.3390/md20110675
Jamee, R., and Siddique, R. (2019). Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. European Journal of Microbiology and Immunology, 9(4), 114–118. https://doi.org/10.1556/1886.2019.00018
Karthi, S., Sangeetha, R. K., Arumugam, K., Karthika, T., and Vimala, S. (2022). Removal of methylene blue dye using shrimp shell chitin from industrial effluents. Materials Today: Proceedings, 66, 1945–1950. https://doi.org/10.1016/j.matpr.2022.05.428
Mazloomi, S., Bonyadi, Z., Haghighat, G. A., Nourmoradi, H., Soori, M. M., and Eslami, F. (2021). Removal of methylene blue by Saccharomyces cerevisiae: Process modelling and optimization. Desalination and Water Treatment, 236, 318–325. https://doi.org/10.5004/dwt.2021.27679
Michael, R. (2024). Outdoor America article. www.iwla.org. https://www.iwla.org/publications/outdoor-america/articles/outdoor-america-2024-issue-2/we’re-running-out-of-clean-water-consumption-contamination-costs
Nabilah, B., Purnomo, A. S., Prasetyoko, D., and Rohmah, A. A. (2023a). Methylene blue biodecolorization and biodegradation by immobilized mixed cultures of trichoderma viride and Ralstonia pickettii into SA-PVA-bentonite matrix. Arabian Journal of Chemistry, 16(8), 104940. https://doi.org/10.1016/j.arabjc.2023.104940
Saravanan, A., Thamarai, P., Kumar, P. S., and Rangasamy, G. (2022). Recent advances in polymer composite, extraction, and their application for wastewater treatment: A Review. Chemosphere, 308, 136368. https://doi.org/10.1016/j.chemosphere.2022.136368
Syala, E., Sadik, W. A., El-Demerdash, A.-G. M., Mekhamer, W., and El-Rafey, M. E. (2024). The effective treatment of dye-containing simulated wastewater by using the cement kiln dust as an industrial waste adsorbent. Scientific Reports, 14(1). https://doi.org/10.1038/s41598-024-64191-5
Zahrim, A. Y., Tizaoui, C., and Hilal, N. (2011). Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A Review. Desalination, 266(1–3), 1–16. https://doi.org/10.1016/j.desal.2010.08.012
Zhang, Z.-H., Xu, J.-Y., and Yang, X.-L. (2021). MXene/sodium alginate gel beads for adsorption of methylene blue. Materials Chemistry and Physics, 260, 124123. https://doi.org/10.1016/j.matchemphys.2020.124123
Zhao, C., Zhou, J., Yan, Y., Yang, L., Xing, G., Li, H., Wu, P., Wang, M., and Zheng, H. (2021). Application of coagulation/flocculation in oily wastewater treatment: A Review. Science of The Total Environment, 765, 142795. https://doi.org/10.1016/j.scitotenv.2020.142795
