Our Class
This course focuses on scientific research and engineering.
During the first part of the year, we conduct independent research
projects that incorporate reviewing literature, making conjectures,
developing methodology, designing experiments, and communicating
findings. Our final projects are presented at a school-wide science
fair, with the possibility for advancement to regional, state, and
international fairs. During the second part of the year, we work in
small teams in order to engineer assistive technology devices. We
meet with clients, conduct patent searches, design and build
prototypes, demonstrate our products to expert judges, and deliver
the products to our clients. Throughout the course, we practice
incorporating purpose, clarity, organization, mechanics, and
audience appeal as they communicate about topics in science and
technology. Assignments consist of research papers, short essays,
technical reports, and presentations.
Background
Invasive organisms reduce biodiversity across the globe,
damaging both ecosystems and species. These exotic animals and
plants often replace the native species through various methods such
as predation, alteration of habitat, disease transmission, and out
competition (Kumar Rai & Singh, 2020). Many invasive plants, like
Kudzu, an invasive Chinese arrowroot, possess the ability to
overshadow entire forests of native species, inhibiting their access
to sunlight, and in turn, deactivating their natural
photosynthesizing ability. Exotic species benefit from their
evolutional abilities, that are in balance with their natural
ecosystem, when relocated into previously uninhabited environments
(Meyer et al., 2021). Lacking predators and parasites, these species
can uncontrollably thrive, throwing off the biological system of
checks and balances, and predators and prey. As climate change
accelerates, the spread of invasive species is expected to
intensify, further disrupting ecosystems, and threatening
biodiversity, making it crucial to address this growing issue now to
safeguard the health of our planet's natural systems. The strongest
natural defense against climate change is biodiversity, only
attained and upheld by the abatement of invasive species
(Biodiversity - our strongest natural defense against climate
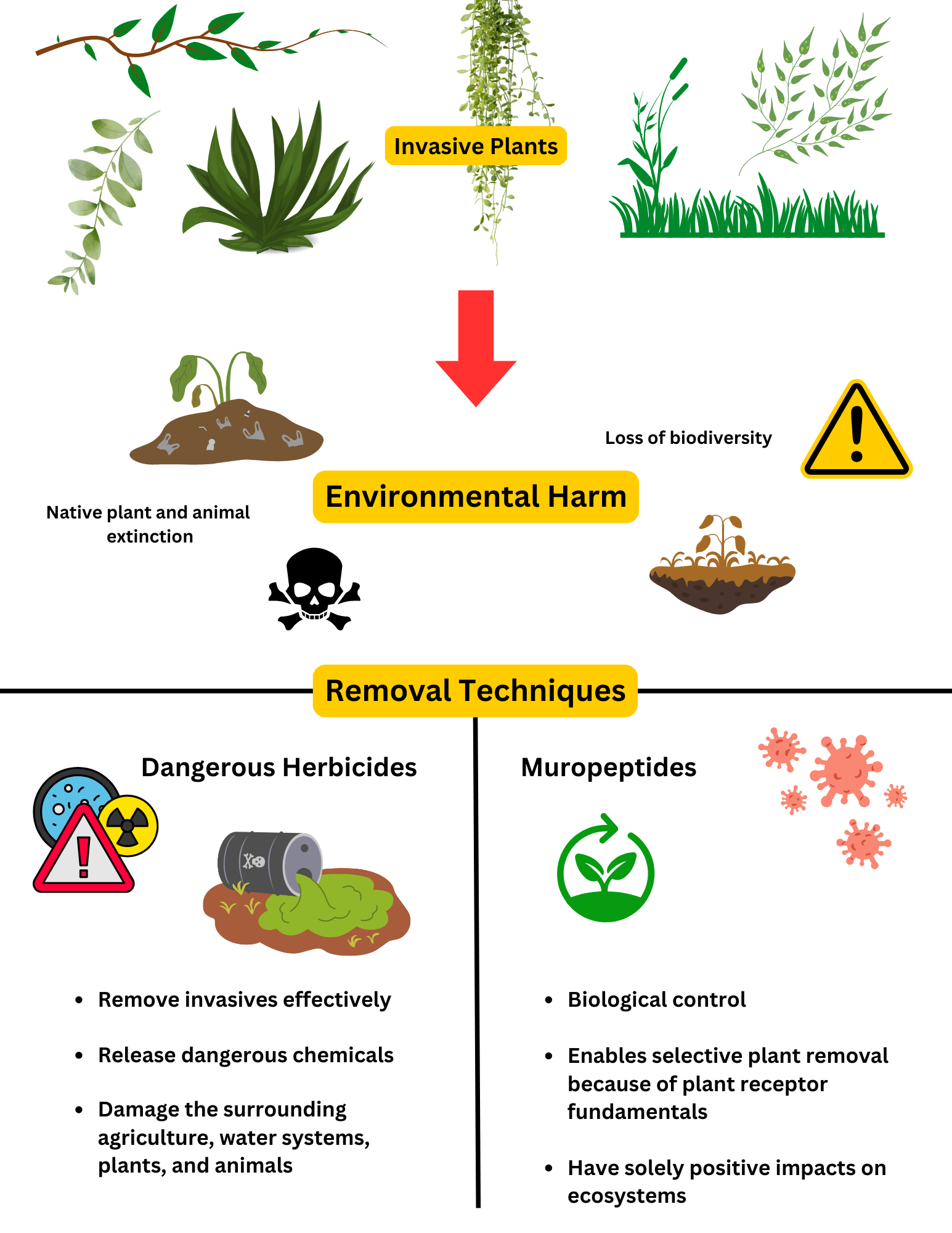
change, n.d.). To limit the invasive plant presence in ecosystems,
several removal techniques exist: mechanical, chemical, and
biological. Mechanical removal involves cutting or pulling the
plants out of the ground and is extremely labor intensive. This
method limits environmental impact but is difficult and exhaustive
to execute. Conversely, using chemicals to eliminate invasive
species is effective and resource-efficient but has the potential to
release toxins into the environment, posing risks to surrounding
wildlife and water systems. Biological removal requires the use of
natural enemies, such as plant diseases or insect predators, that
will either outcompete the invasive species or directly target it.
However, biological removal can have inadvertent consequences and
requires high amounts of research on all possible outcomes as to not
introduce control agents that can harm non-target species and
disrupt the balance of the ecosystem, potentially resulting in
further loss of biodiversity (Pearson et al., 2021). Both chemical
and biological removal have the potential to drive hundreds of
species to extinction if not used properly and with thorough
testing. Many of the herbicides used in killing invasive plant
species damage surrounding native plants and have immense negative
environmental impacts. There is no established method capable of
effectively eliminating invasive plants while simultaneously
preserving the health of surrounding species. This research seeks to
investigate the escalating expansion of invasive, non-native plant
species that have caused widespread degradation of the environment,
water systems, agriculture, biodiversity, and essential ecosystem
services in regions worldwide. Muropeptides are a main component of
bacterial cell wall peptidoglycan, which is a crucial structural
component that provides rigidity and strength in bacteria and helps
them protect against osmotic pressure. Peptidoglycan receptors have
been identified in some plant species, but very little research has
been conducted on how they work (Andrea A. Gust, 2015). However, it
has been established that different plant immune responses are
linked to variations in the carbohydrate or peptide parts of the
peptidoglycan (Erbs et al., 2008). Observing the effects of
peptidoglycan on plants, this research has the potential to address
the substantial environmental damage caused by invasive, exotic
species across the globe. By gaining a deeper understanding of the
mechanisms behind their spread and impact, this work could lead to
more effective strategies for mitigating their disruptive effects on
ecosystems. Invasive species are known to threaten biodiversity,
alter habitats, and disrupt ecological balance, and thus, efforts to
control and prevent their development could have profound benefits
for the preservation of natural environments and the health of
ecosystems worldwide.
References
Crystal-Ornelas, R., Hudgins, E. J., Cuthbert, R. N., Haubrock, P.
J., Fantle-Lepczyk, J., Angulo, E., Kramer, A. M., Ballesteros-Mejia,
L., Leroy, B., Leung, B., López-López, E., Diagne, C., & Courchamp,
F. (2021). Economic costs of biological invasions within North
America.
NeoBiota, 67(67), 485–510.
https://doi.org/10.3897/neobiota.67.58038
Erbs, G., Silipo, A., Aslam, S., De Castro, C., Liparoti, V.,
Flagiello, A., Pucci, P., Lanzetta, R., Parrilli, M., Molinaro, A.,
Newman, M. A., & Cooper, R. M. (2008). Peptidoglycan and muropeptides
from pathogens
Agrobacterium and
Xanthomonas elicit
plant innate immunity: structure and activity.
Chemistry &
Biology, 15(5), 438–448.
https://doi.org/10.1016/j.chembiol.2008.03.017
Gust, A. A., Biswas, R., Lenz, H. D., Rauhut, T., Ranf, S.,
Kemmerling, B., Götz, F., Glawischnig, E., Lee, J., Felix, G., &
Nürnberger, T. (2007). Bacteria-derived Peptidoglycans Constitute
Pathogen-associated Molecular Patterns Triggering Innate Immunity in
Arabidopsis.
Journal of Biological Chemistry, 282(44),
32338–32348.
https://doi.org/10.1074/jbc.m704886200
Meyer, S. E., Callaham, M. A., Stewart, J. E., & Warren, S. D.
(2021). Invasive Species Response to Natural and Anthropogenic
Disturbance. In
Invasive Species in Forests and Rangelands of
the United States, 85–110.
https://doi.org/10.1007/978-3-030-45367-1_5
Patten, K., O'Casey, C., & Metzger, C. (2017). Large-Scale Chemical
Control of Smooth Cordgrass (
Spartina alterniflora) in Willapa
Bay, WA: Towards Eradication and Ecological Restoration.
Invasive
Plant Science and Management, 10(3), 284–292. doi:
10.1017/inp.2017.25
Pearson, D. E., Clark, T. J., & Hahn, P. G. (2021). Evaluating
unintended consequences of intentional species introductions and
eradications for improved conservation management.
Conservation
Biology.
https://doi.org/10.1111/cobi.13734
Qiao, P., Wang, A., Xie, B., Wang, L., Han, G., Mei, B., & Zhang, X.
(2019). Effects of herbicides on invasive Spartina
alterniflora in the Yellow River Delta. Acta Ecologica
Sinica, 39(15), 5627–5634.
Willmann, R., Lajunen, H. M., Erbs, G., Newman, M.-A., Kolb, D.,
Tsuda, K., Katagiri, F., Fliegmann, J., Bono, J.-J., Cullimore, J.
V., Jehle, A. K., Gotz, F., Kulik, A., Molinaro, A., Lipka, V., Gust,
A. A., & Nurnberger, T. (2011). Arabidopsis lysin-motif proteins
LYM1, LYM3, CERK1 mediate bacterial peptidoglycan sensing and
immunity to bacterial infection.
Proceedings of the National
Academy of Sciences, 108(49), 19824–19829.
https://doi.org/10.1073/pnas.1112862108
Xie, B., Han, G., Qiao, P., Mei, B., Wang, Q., Zhou, Y., Zhang, A.,
Song, W., & Guan, B. (2019). Effects of mechanical and chemical
control on invasive
Spartina alterniflora in the Yellow River
Delta, China.
PeerJ, 7, e7655.
https://doi.org/10.7717/peerj.7655