STEM (Independent Research Project)
Each student works on an Independent Research Project for five months. Students can choose projects of their liking, ranging from psycological studies, biology projects, projects involving machine learning, or engineering projects. Students start with in-depth research and then move on to data collection and finally write a thesis for the class. Students take part in the science fair and may move up to WRSEF. This course is taught by Dr. Kevin Crowthers, also know as the STEM Overlord.
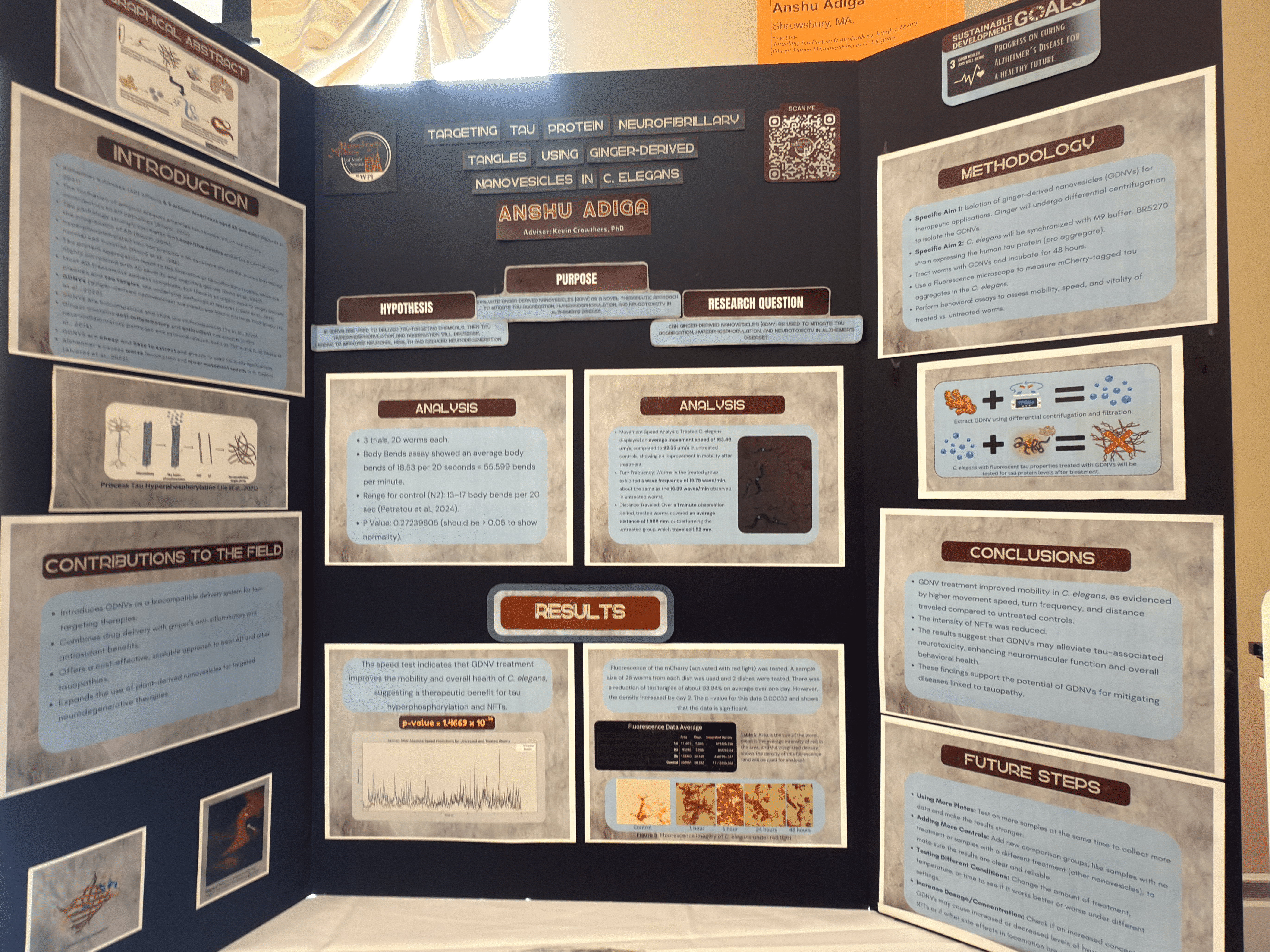
Targeting Tau Protein Neurofibrillary Tangles Using Ginger-Derived Nanovesicles in C. Elegans
Alzheimer's disease is a neurodegenerative disorder that affects millions of people worldwide. Although current "treatments" are to manage the symptomps such as dementia or slow movment, not many breakthroughs have been made in targeting the root cause: β-amyloid plaques and tau protein tangles. This project specifically focuses on tau tangles, as research has shown that tau tangles can aggregate Aβ plaques. The project showed that worms' speeds improved significantly after treatment with Ginger-Derived Nanovesicles (GDNV)
Abstract
People with neurodegenerative diseases, which can lead to dementia, have trouble performing everyday tasks. Alzheimer's disease (AD) is a neurodegenerative disorder defined by the accumulation of amyloid-beta plaques and neurofibrillary tangles, which lead to cognitive decline and neuronal dysfunction. Recent research has focused on plant-derived nanovesicles, particularly ginger-derived nanovesicles (GDNVs), as a novel platform for drug delivery to the brain. GDNVs are membrane-bound particles, that show biocompatibility and low immunogenicity. Ginger (Zingiber officinale) is an anti-inflammatory rhizome and has antioxidant properties and provides bioactive molecules like 6-gingerol and 6-shogaol, which have shown promise in reducing neuroinflammation and oxidative stress—two key contributors to AD pathogenesis (Matin et al., 2024). This project investigates the potential of GDNVs as a targeted drug delivery system for AD. Preliminary locomotion assay data from a wildtype strain of C. elegans (N2) showed a significant increase in speed, but more data is needed to get conclusive results. These results highlight the potential of GDNVs to improve the treatment of Alzheimer’s disease by enhancing neuroprotection and slowing disease progression.
Graphical Abstract
Research Question
Evaluate ginger-derived nanovesicles (GDNV) as a novel therapeutic approach to mitigate tau aggregation, hyperphosphorylation, and neurotoxicity in Alzheimer’s disease using C. elegans as a model.
Hypothesis
If GDNVs are used to deliver tau-targeting therapies, then tau hyperphosphorylation and aggregation will decrease, leading to improved neuronal health and reduced neurodegeneration in C. elegans, because GDNVs enhance the bioavailability and stability of therapeutic agents while reducing inflammation and oxidative stress.
Background
- Alzheimer’s disease (AD) affects 6.9 million Americans aged 65 and older (Rajan et al., 2021).
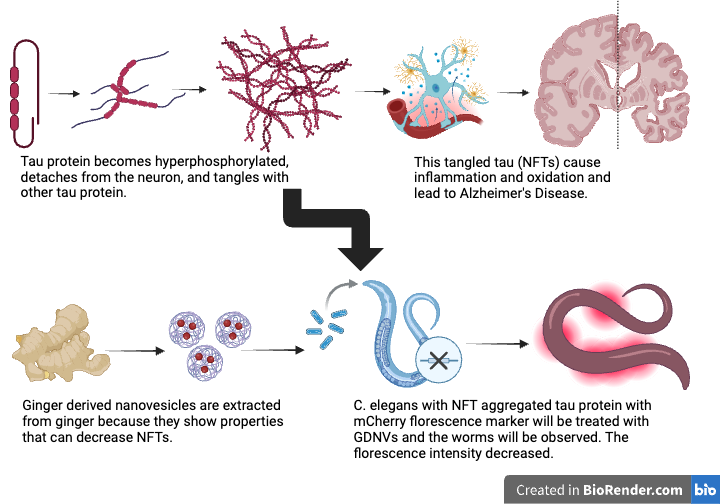
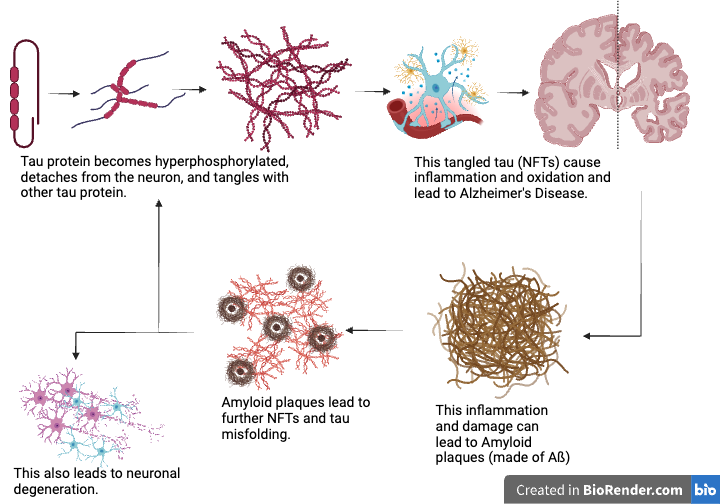
- The formation of amyloid plaques amplifies tau tangles, which are primary contributors to AD pathology (Bloom, 2014).
- Tau pathology strongly correlates with cognitive decline and plays a central role in the progression of AD (Bloom, 2014).
- Hyperphosphorylated tau: tau proteins with excessive phosphate groups that disrupt normal cell function (Wood et al., 1986).
- Tau protein aggregation leads to the formation of neurofibrillary tangles, which are highly correlated with AD severity and cognitive decline (Guha et al., 2020).
- Most AD treatments address symptoms, but there is an urgent need to target amyloid plaques and tau tangles, the underlying pathological features (Lahiri et al., 2013).
- GDNVs (ginger-derived nanovesicles) are membrane-bound particles from ginger (Yu et al., 2020).
- GDNVs are biocompatible and show low immunogenicity (Yu et al., 2020).
- Ginger contains anti-inflammatory and antioxidant compounds that inhibit neuroinflammatory pathways and cytokine release, such as TNF-α and IL-1β (Wang et al., 2014).
- GDNVs are cheap and easy to extract and are already used for many applications.
- Alzheimer’s causes worse locomotion and lower movement speeds in C. elegans (Alvarez et al., 2022).
- 6-gingerol and 6-shogaol are bioactive compounds in ginger that have shown promise in reducing neuroinflammation and oxidative stress (Matin et al., 2024).
- How does this contribute to the field?
- Introduces GDNVs as a biocompatible delivery system for tau-targeting therapies.
- Combines drug delivery with ginger’s anti-inflammatory and antioxidant benefits.
- Offers a cost-effective, scalable approach to treat AD and other tauopathies.
- Expands the use of plant-derived nanovesicles for targeted neurodegenerative therapies.
Methods
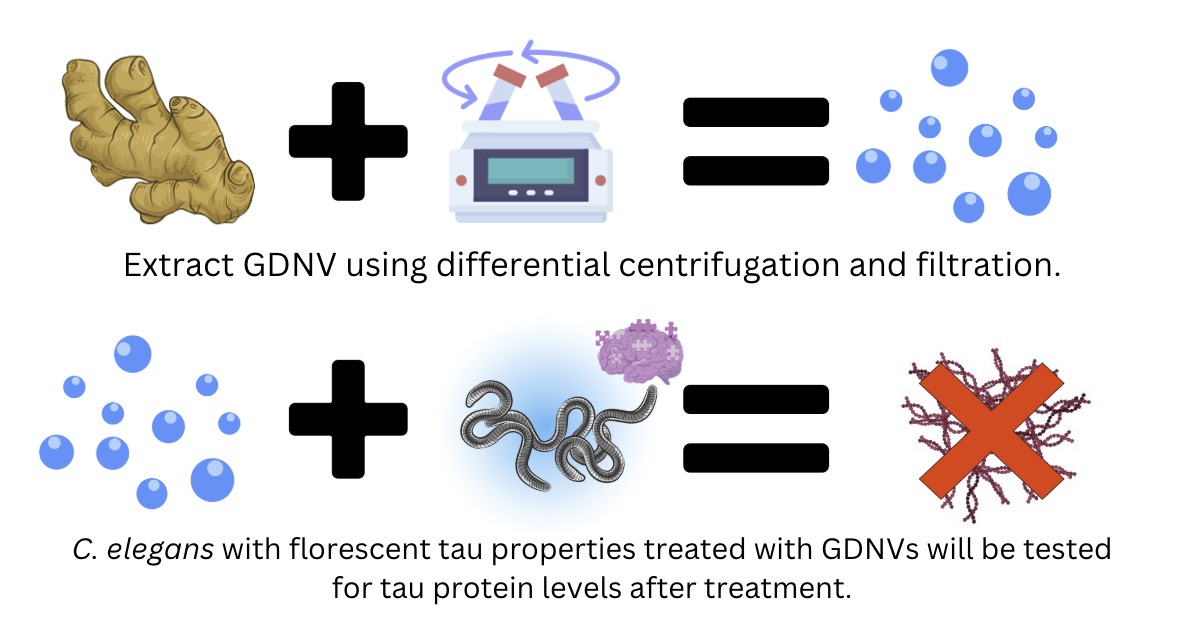
- Specific Aim 1: Isolation of ginger-derived nanovesicles (GDNVs) for therapeutic applications. Ginger will undergo differential centrifugation and filteration to isolate the GDNVs.
- Specific Aim 2: C. elegans will be synchronized with M9 buffer. BR5270 strain expressing the human tau protein (pro aggregate) will be used as experimental group.
- Treat worms with GDNVs and incubate for 48 hours.
- Use a fluorescence microscope to measure mCherry-tagged tau aggregates in the C. elegans.
- Perform behavioral assays to assess motility, speed, and vitality of treated vs. untreated worms.
Results
Discussion
Conclusion
This study demonstrates the potential of ginger-derived nanovesicles (GDNVs) as a
novel therapeutic approach for targeting tau aggregation in Alzheimer’s disease. The results
suggest that GDNVs may help reduce tau accumulation in C. elegans, leading to improved
locomotion. These findings support the hypothesis that GDNVs can serve as an effective delivery
system for tau-targeting therapies, leveraging their anti-inflammatory and neuroprotective
properties to mitigate neurodegeneration. Since slower locomotion and tau tangles are attributed
to AD, this suggests that GDNVs can help reduce the likely cause of AD.
Further research is needed to refine the use of GDNVs in mammalian models and to determine their
long-term effects on tau pathology. If successful, GDNV-based therapies could provide a
biocompatible, cost-effective, and scalable treatment option for Alzheimer’s and other
tauopathies. This study contributes to the growing field of nanovesicle-based drug delivery and
highlights the potential of plant-derived nanotechnology in neurodegenerative disease treatment.
References
Acknowledgements
Special thanks to Dr. Kevin Crowthers for his guidance and support throughout this project. I would also like to thank my classmates for their feedback and encouragement. This project would not have been possible without the help of my family and friends. Additionally, Vaishnavi, Hasini, Ila, Saketh, Mary, and Jasmin provided support with the preparation and set up for materials relating to the C. Elegans model species work in the lab.