ES 3001 Thermodynamics
Mechanical Engineering Department
Worcester Polytechnic Institute
Introduction
Staffing
Course Objectives
Grading Policy
Thermodynamics
Staffing
Course Coordinator:
Professor John M. Sullivan, Jr.
Office: HL 111
Office Phone 831-5199
Email: sullivan@wpi.edu (Used extensively!)
Teaching Assistants for the Term:
Textbook:
Assigned: Moran and Shapiro, "Fundamentals of Engineering Thermodynamics", 7th Edition, John Wiley Press. (Most figures displayed on this website are taken from the required textbook). Earlier versions of the textbook will work equally well for the course.
Course Objectives:
Thermodynamics is both a branch of science and a specialty within engineering.
In this course you will be introduced to properties of matter (Temperature, Pressure, Enthalpy, Specific Volume, Entropy, etc.) and governing laws which can be used to describe the behavior of matter and its interaction with the surrounding environment.
You will learn how to identify systems and to use thermodynamic analysis to describe the behavior of the system in terms of properties and processes. Understanding how to apply these concepts is a powerful tool for the engineer, enabling the evaluation of material states (phases) under different conditions and the maximum efficiency achievable with various power cycles.
After completing this course, you should be able to define and describe properties and processes used in thermodynamic analysis and the governing laws. In addition you should be able to apply control volume analysis and thermodynamic principles to solve problems involving power and refrigeration cycles.
**************
Please note: If you need course adaptations or accommodations because of a disability, or if you have medical information to share with me that may impact your performance or participation in this course, please make an appointment with me as soon as possible.
If you have approved accommodations, please go to the Exam Proctoring Center (EPC) in Morgan Hall to pick up Letters of Accommodation and let me sign them.
If you have not already done so, students with disabilities who need to utilize accommodations in this class are encouraged to contact the Office of Disability Services (ODS) as soon as possible to ensure that such accommodations are implemented in a timely fashion. This office can be contacted via email: DisabilityServices@wpi.edu, via phone: (508) 831-4908, or in person: 137 Daniels Hall.
*************
Grades will be based on:
60%
for Exams (20% each)
4 Exams, drop lowest one (Exam Dates!)
40% for Homework and In-Class Assignments
All homework assignments and exams are done by each individual without collaboration.
Please review the Judicial Flow for suspected Academic Dishonesty violations at http://www.wpi.edu/offices/policies/honesty.html
Science dealing with how Matter behaves in relation to heat and work exchanged with the surroundings.
Phases of Matter - Solid (ES2001 Materials), Liquids (ES3001), Gases (ES3001), Plasma (Graduate School)
Applications of Thermodynamics: (See Table 1.1 of text - huge!)


The Design and Analysis of many engineering systems requires thermodynamics (in addition to fluid mechanics, heat transfer, structural analysis, etc.). Another short description of Thermodynamics is:
Consider a Simple Power System. Thermodynamics establishes the limits on the Maximum Power Out (Work Out) for a given Qin for a particular system of interest.
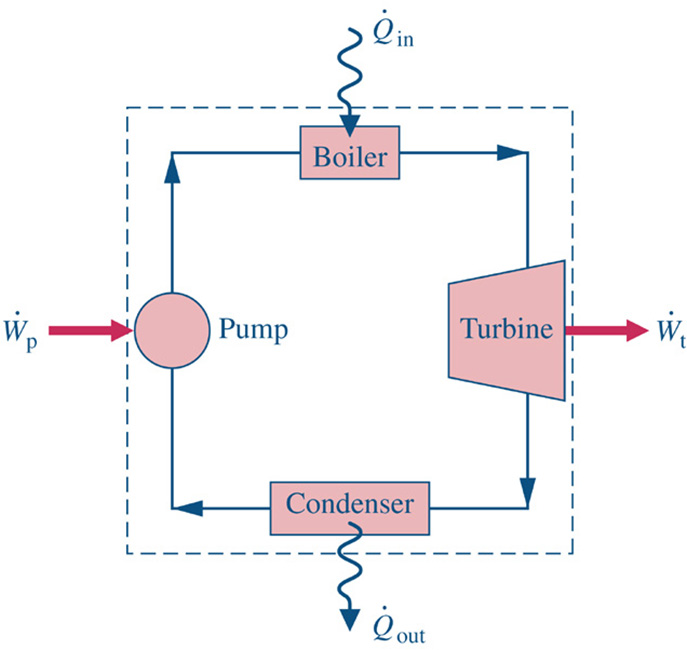
System: Defines what matter is under study.
Surroundings: Everything external to the System.
Boundary: The invisibly thin perimeter that separates the System from the Surroundings.

Closed System: Fixed mass system. No change in mass. No flow of mass across boundary.
Isolated System: No exchange with surroundings of any kind (no mass-, no heat-, no work- exchange).
Control Volume: A region delimited by a boundary across which mass flows.


Classical Thermodynamics: Macroscopic viewpoint (i.e. This Course!) Deals with averaged properties, not molecular motion items.
Statistical Thermodynamics: Microscopic viewpoint (i.e. Not this course) Deals with thermodynamic properties interperted through molecular behavior. It requires statistical techniques to obtain observables (such as P, T, etc.).
Properties: Macroscopic characteristics of a system (mass, volume, energy, pressure, temperature).
State: Conditions of a system as defined by a specific set of properties.
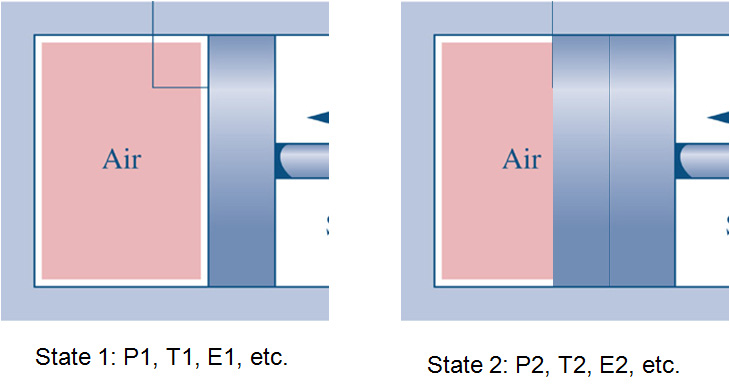
Process: Transformation from one state to another (such as the piston compressing the air).
Steady State: Conditions of a system if none of its properties are changing with time.
Thermodynamic Cycle: A sequence of processes that begins and ends at the same state.

Intensive Property: (also called a bulk property), is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant. T, P, v (Degrees, Force per Unit area, Volume per Unit mass).
Extensive Property: is a physical property of a system that DOES depend on the system size or the amount of material in the system: V, E (Volume, Energy).
Convention: The literature uses upper case for Extensive Properties and lower case for the intensive property (generally). (T and P are exceptions.)
Units: Review chapter 1 (and homework problems).
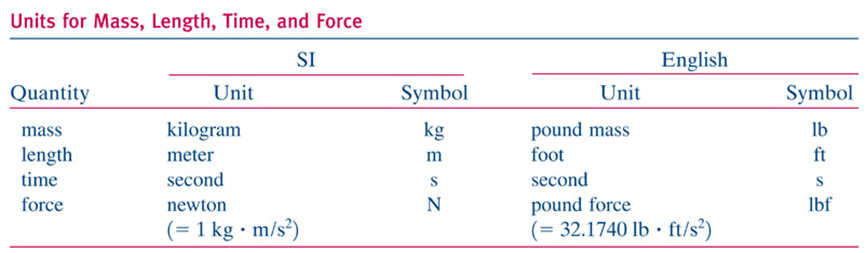
Ex: Power in Watts, J/s
Power = work / time
Work = Force * Distance
Force = ma = kg m/s2
work = kg m2/s2
power = kg m2/s3
= Watt (Force * Length / time)
Specific Volume: (i.e. the reciprocal of density) It is the volume per unit mass.
Pressure: has units of force per unit area.
Temperature: Measure of kinetic energy of matter on a microscopic scale. Temperature scales are Centigrade (deg. C), Kelvin (K), Farenheit (deg. F), and Rankin (R).
T(degC) = T(K) - 273.15;
T(K) = T(degC) + 273.15;
T(degF) = T(R) - 459.67;
T(R) = T(degF) + 459.67;
T(degC) = (5/9) (T(degF) - 32)
T(degF) = (9/5) T(degC) + 32
Zeroth Law of Thermodynamics: If two objects are in thermal equilibrium with a third object, then they are in equilibrium with each other. (This is the basis of a thermometer usage.)
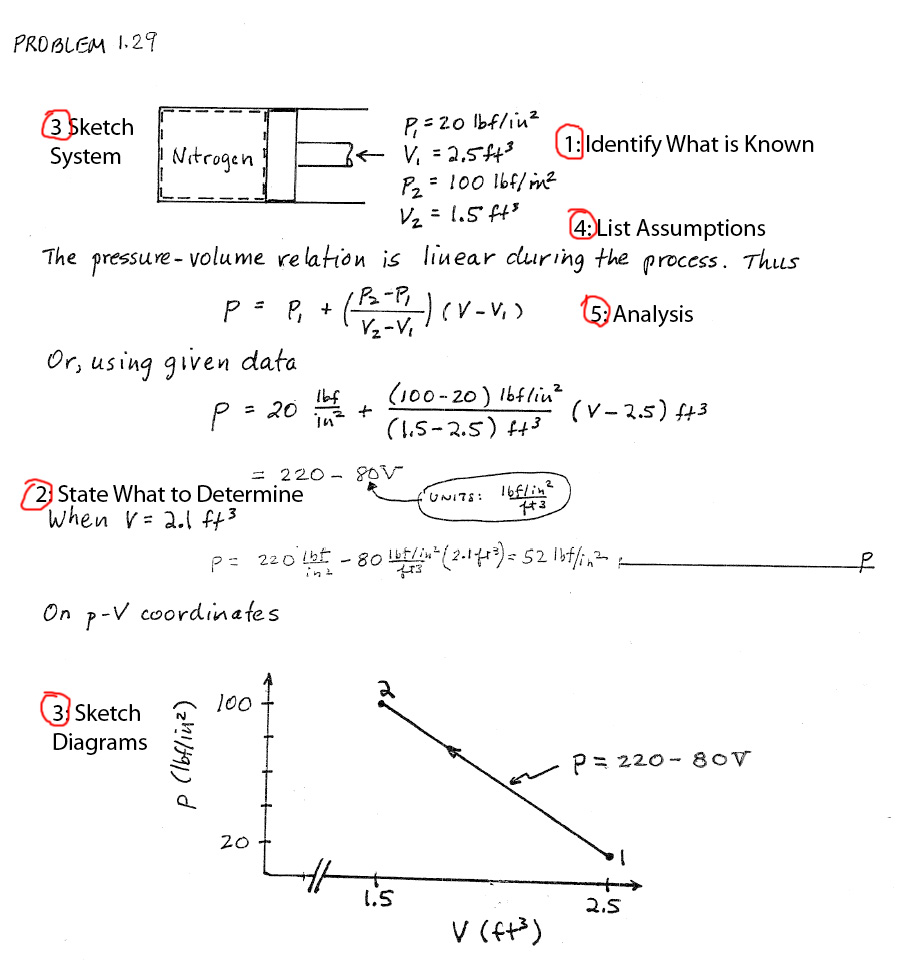
Systematic Approach to Solve Thermodynamics Problems:
1) Identify what is known. (Do this carefully!)
2) State what is to be determined.
3) Sketch System - pay attention to Boundary and Surroundings. Sketch any property diagrams (T vs h), (P vs v), etc.
4) List simplifications and idealizations -> constitutes a 'model'.
5) Analysis - using relevant conservation laws or governing relationships (gas laws, process relationships, etc.). Substitute numerical values (ONLY at the end, and keep all units linked with the variables and numbers!).
Examples: