Dr. Arne Gericke
John C. Metzger Professor
Worcester Polytechnic Institute
Department of Chemistry & Biochemistry
Dr. Arne Gericke
John C. Metzger Professor
Worcester Polytechnic Institute
Department of Chemistry & Biochemistry













Our laboratories are located in the Life Sciences and Bioengineering Center (LSBC) at Gateway Park. The building hosts several core facilities (imaging core facility, NMR spectroscopy and x-ray facility, vivarium) and excellent facilities for cell and tissue culture studies as well as protein expression. Our well funded research groups have additional equipment in their respective labs. Workshops can be found on the main campus, which is a short walk from Gateway Park. Students have access to a broad range of scientific software packages and excellent computing facilities.
LSBC Core Facilities

Gericke Group Major Instrumentation
In addition to several small lab instruments that are being used for sample preparation (e.g., centrifuges, balances, high power sonicator, pH meter, vesicle extrusion kit), our lab is equipped with a broad range of high-end instrumentation for the characterization of lipid phase behavior, membrane morphology and lipid/protein interactions as well as an infrared microscope for the characterization of tissue and surfaces. Please contact Dr. Gericke or any of the lab members if you wish to use the equipment.
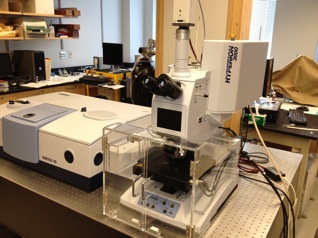
Bruker Hyperion FTIR Microscope with Focal Plane Array Detector
Hyperion 3000 microscope with a 128 x 128 pixel Focal Plane Array (FPA) detector. The field of view of the detector is about 250 x 250 um. For 4 cm-1 spectral resolution, a full array with 16384 spectra is generated in about 5 minutes. The spatial resolution is restricted by the wavelength of the infrared radiation, i.e., a spatial resolution of a few microns can be achieved. The instrument can also require basic fluorescence images that can be overlaid with the IR image. We have several objectives available, including a 15x and 36x objective for transmission microscopy as well as attenuated total reflection (ATR) and grazing incidence reflection (GIR) objectives for the analysis of surfaces. All measurements can be carried out with polarized radiation.

Bruker Equinox FTIR with A-511 reflection unit for infrared reflection-absorption spectroscopy (IRRAS) of monolayers at the air/water interface
Dr. Gericke has been one of the pioneers of this technique. The instrument is equipped with Langmuir trough that consists of a sample and reference trough. The reflection unit can automatically shuttle between the two troughs to acquire sample and reference spectra. The angle of incidence (200 - 850) as well as the polarization state (s- and p-polarization) can be changed throughout the experiment using customized software. For a description of the IRRAS technique, see R. Mendelsohn, J. Brauner, A. Gericke (1995) “External infrared reflection-absorption spectrometry of monolayer films at the air/water interface” Ann. Rev. Phys. Chem. 46: 305 - 334. Journal


Bruker IFS 66/S FTIR Spectrometer
This high-end step-scan/rapid scan FTIR spectrometer is capable of measuring with a spectral resolution better than 0.1 cm-1. In the rapid scan mode, 100 spectra/sec. can be acquired, while in the step-scan mode the temporal resolution is 10 ns. The instrument is equipped with a mid-range MCT detector (holding time ~ 18 hours).
Bruker Tensor 27 FTIR Spectrometer
This “work horse” spectrometer can be used for a variety of applications ranging from transmission to grazing incidence and attenuated total reflection spectroscopy. Our accessories that can be used for any of the IR spectrometers, include: thermostated IR transmission cell (temperature software controlled), overhead ATR unit (Bruker A 537), confocheck BioATR cell, AutoSeagull (automated external reflection accessory), electrophoresis ATR.

Applied Photophysics SX20 Stopped-Flow Spectrometer
The SX20 stopped flow spectrometer can be used to study transient and pre-steady state kinetics of fast, liquid-phase chemical and biochemical reactions initiated by the rapid mixing and stopping (stopped-flow) of the reactants. The instrument is operated in the fluorescence mode (excitation from the UV to red) and is capable of measuring reaction rates up to 2000 s-1. The instrument can measure simultaneously the temporal evolution of two fluorescence emission intensities
Reichert Surface Plasmon Resonance System (SPR)
The Reichert SR7500 instrument is a dual channel SPR instrument that can be used to study the interactions (binding) of a broad range of biomolecules, including proteins, nucleic acids, lipids, carbohydrates, small molecules (Mw > 80 g/mol) and even whole cells and viruses. The accessible affinities range from extremely weak (1 mM) to very strong (1 pM). The temperature range of the unit is from 100C below ambient to 700C. The unit is run with an autosampler that provides space for 768 samples.

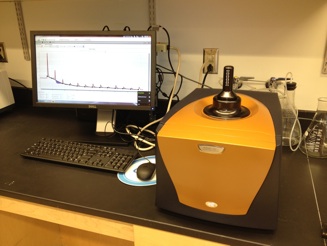
TA Instruments Isothermal Titration Calorimeter (ITC)
This low volume nano ITC allows to study the interaction of a large variety of binding partners. The instrument provides access to thermodynamic parameters like free enthalpy, enthalpy, entropy and binding constant.

Malvern Zetasizer Nano ZS
This dynamic light scattering (DLS) instrument has a measurement range of 0.3 nm - 10.0 microns (diameter). In addition, it is possible to measure with the instrument the zeta potential (surface charge) of the respective particles

Fluorescence Microscopy of Monolayers at the Air/Water Interface
This Olympus BX51 microscope is equipped with a Hamamatsu EM CCD camera and a NIMA 601M Langmuir trough. The setup is used to study the morphology of monolayers at the air/water interface. A water immersion 60x objective can be used for traditional fluorescence microscopy measurements.